Introduction
An abdominal hernia can occur following any operation involving an incision of the abdominal wall. This complication is relatively common, with a reported incidence of 2% to 20% in patients who have undergone open abdominal surgery. However, appropriately managing an abdominal hernia can be challenging due to the need to account for several factors, such as the patient’s comorbidities, the morphological characteristics of the hernia, and the wide range of possible surgical options [1].
Although several studies have described repair methods for abdominal hernia, studies focusing on hernia repair with acellular dermal matrix (ADM) are rare. Among them, only two cases used multilayer ADM [2,3]. Xiao et al. [2] used a single layer of ADM to cover a hernia; vascularization and adhesion to the wound bed were noted 8 days later, after which they added another layer of ADM. Kolker et al. [3] advanced the rectus muscle after placing one layer of ADM as an underlay to the retrofascia of the rectus muscle with myofascial release and covered it with another layer as an overlay after repair. In our case, we describe a case of hernia after wide resection of fibrosarcomatous dermatofibrosarcoma protuberans (DFSP-FS) in the abdominal wall, which was repaired using multilayer ADM and a split-thickness skin graft. The patient provided written informed consent for the publication and the use of his images.
Case
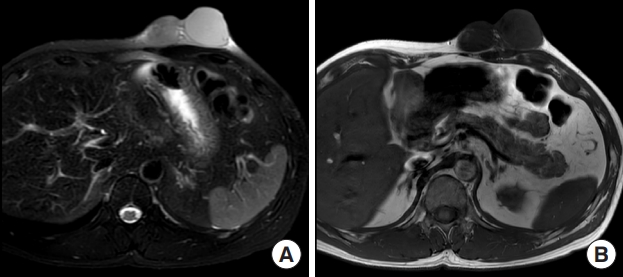
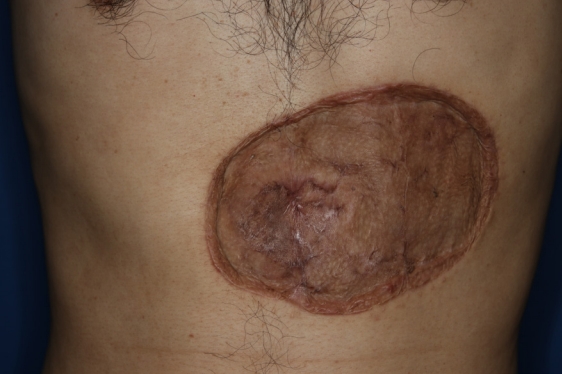
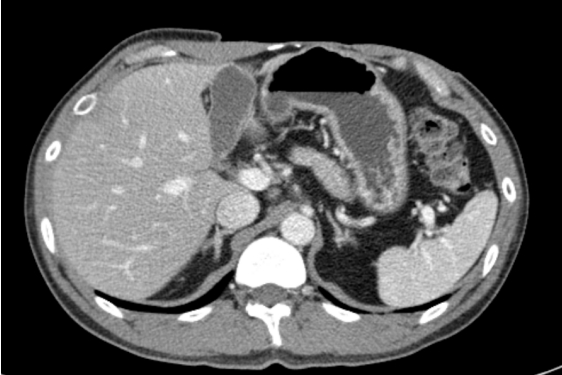
A 41-year-old man presented at our outpatient clinic due to a palpable abdominal mass that had developed 4 years prior. He reported a sudden growth of the mass 4 months before the visit (Fig. 1). Magnetic resonance imaging (MRI) revealed a 7.8×6.4×4.2 cm lobulated mass with peripheral vascularity, suggesting soft tissue sarcoma suspected to be angiosarcoma in the subcutaneous layer of the left anterior upper abdominal wall (Fig. 2). Positron emission tomography/computed tomography showed no signs of lymph node involvement or distant metastasis. The patient had no notable past medical history or family history.
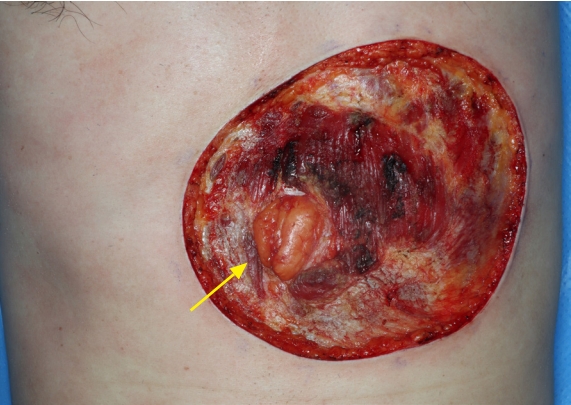
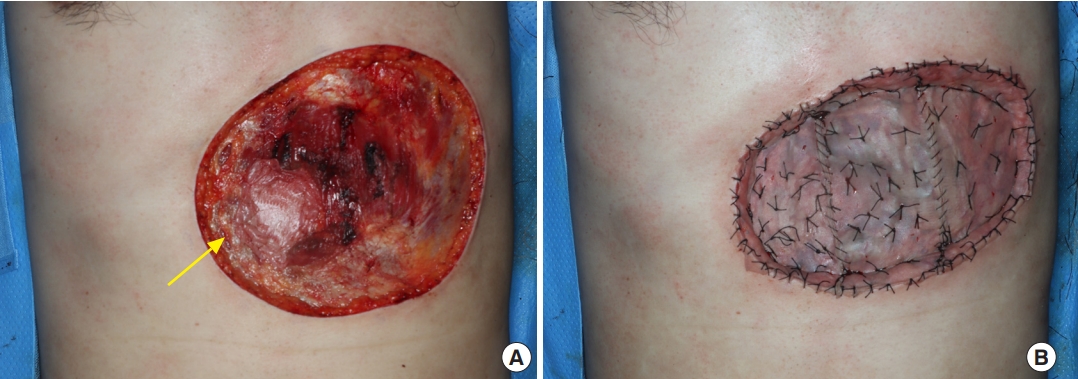
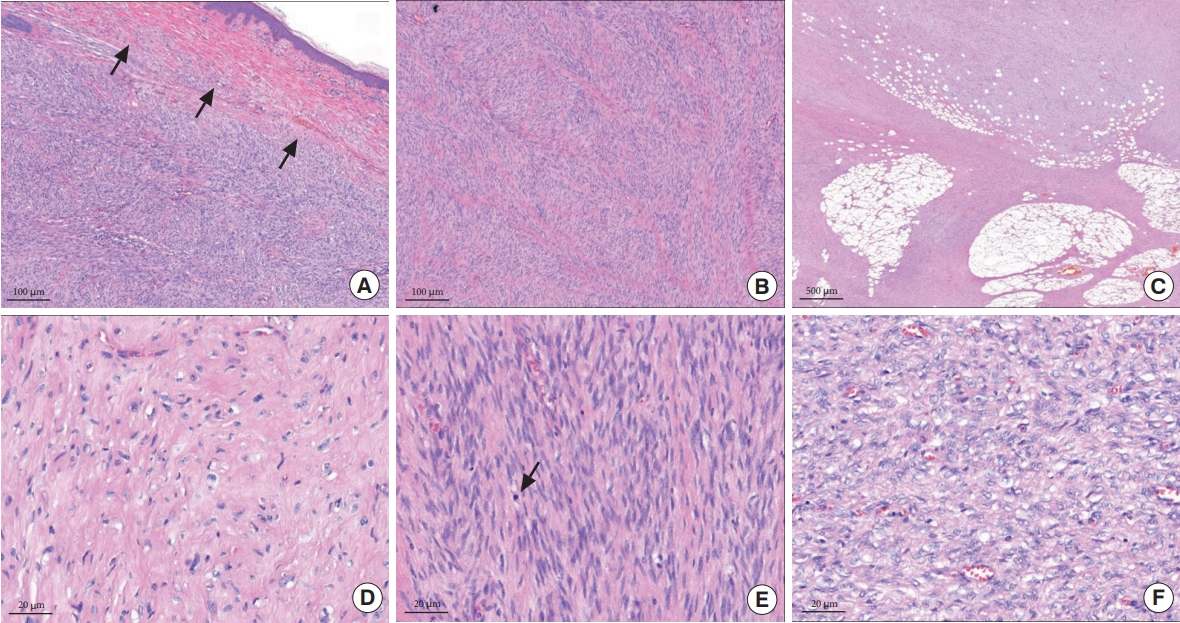
Under general anesthesia, wide excision was performed with a 3-cm circumferential safety margin. The frozen biopsy performed during surgery showed tumor positivity in the fascia, where the base of the mass was located. Another frozen biopsy was performed after partially removing the rectus muscle below it, and tumor positivity with atypical cells was suspected. Therefore, a full-layer removal of the underlying rectus muscle was performed. Frozen biopsy was then performed on the remaining margins of the removed rectus muscle, and tumor cell negativity was confirmed. After wide local excision, a 6.0×5.8 cm hernia was identified along the rectus abdominis muscle (Fig. 3). ADM (MegaDerm, L&C BIO Inc.) was overlaid on the hernia in four layers using Vicryl 4-0, and a 0.2-mm thick split-thickness skin graft was applied over the multilayer ADM using both thighs as donors (Fig. 4). The neoplastic cells of the tumor were negative for desmin, actin, and S-100 on immunohistochemistry, but were immunoreactive for CD34, a stem cell marker. The Ki-67 index, a cell proliferation indicator, was 5% to 10%. The mass was confirmed to be DFSP-FS based on histopathological and immunohistochemical examination (Fig. 5). The malignant tumor was designated as stage IIA according to the modified staging system of dermatofibrosarcoma protuberans (DFSP) [4]. The resected margins did not contain any tumor cells. During a 10-month follow-up period, no recurrence or metastasis of the mass was noted. The hernia also did not recur (Figs. 6, 7).
Discussion
Complete surgical resection with histologically negative margins is the optimal treatment for resectable DFSP [4-6]. Surgical options for such treatment include wide local excision to obtain negative tumor margins, Mohs micrographic surgery, and amputation for tumors occurring in the upper or lower digits [6]. We performed a wide local excision with a safety margin of 3 cm, taking into account tumor location, cost, surgical time, and immediate reconstruction. In collaboration with the pathology department, it was confirmed that no tumor cells remained. After the tumor was removed, a hernia was observed along the rectus abdominis muscle.
Although the treatment of choice for reconstructing abdominal wall defects accompanied by hernias has not yet been established, various reconstruction methods can be considered depending on the presence of contamination, the depth of the defect (skin loss, fascia loss, and myofascial loss), and defect size [7]. The possible methods include a simple locoregional flap, synthetic or biological mesh repair, and functional free flap reconstruction. The patient described in this report was considered to have a clean wound, from which part of the rectus abdominis muscle and fascia was unilaterally removed. Thus, it would have made sense to consider repairing the hernia using synthetic or biological mesh and reconstructing the defect using a locoregional flap. However, the authors chose to perform hernia repair and one-stage reconstruction with a skin graft because the preoperative chest MRI findings (peripheral vascularity with well-enhanced, slightly high T1 and high T2 signals) were suggestive of angiosarcoma. Cutaneous angiosarcoma is a very infrequent condition, and relatively few clinical experiences with this tumor have been described. Therefore, no consensus exists regarding treatment strategies [8]. Nonetheless, the vascular origin of angiosarcoma may be linked to a poor prognosis, and hematogenous spread of angiosarcoma also occurs frequently [9]. This caused us to be concerned about the potential for recurrence through hematogenous spread if reconstruction had been performed using a vascular pedicle. Furthermore, given the high recurrence rate of angiosarcoma, a split-thickness skin graft from both thighs was performed, taking into account the likelihood of additional surgery due to recurrence.
For hernia repair, we determined that ADM would be more appropriate than permanent prosthetic mesh. Although permanent prosthetic mesh is deemed to be the gold standard in terms of minimizing hernia recurrence and cost, ADM represents an intriguing biological alternative [10]. It can be used in contaminated surgical fields because it supports tissue regeneration, with concomitant revascularization, fibroblast repopulation, collagen deposition, and eventual absorption and replacement with native fascia [11]. After the application of ADM to a full-thickness skin defect, onto which a very thin split-thickness skin graft has been placed, cells migrate into the ADM from the peripheral skin margin, underlying tissue, and the skin graft [12]. ADM also has the ability to increase the thickness of the dermis and enhance the acceptability of outcomes at subcutaneous tissue defect sites and at sites of tendon or cartilage exposure [9]. Generally, a single layer of ADM is used to perform an allograft, followed by a skin graft, but we wanted to reinforce the abdominal wall more firmly with a multilayer ADM. To the best of our knowledge, two studies have described reconstructing an abdominal wall defect accompanied by a hernia using a multilayer ADM. Xiao et al. [2] used a single layer of ADM to cover a hernia. Eight days later, vascularization and adhesion to the wound bed were observed, and the authors then placed another layer of ADM. Kolker et al. [3] advanced the rectus muscle through the placement of a single-layer allograft beneath the retrofascia of the rectus muscle. They implemented myofascial release and, following the repair, added an additional layer as an overlay. Subsequently, they performed subcutaneous tissue and skin closure. Unlike those two studies, the musculofascial layer was not advanced in this case. Since a single-stage operation was performed, a multilayer ADM was used as an overlay to firmly support the abdominal wall.
DFSP has numerous histological variants, encompassing atrophic, granular cell, myxoid, and pigmented DFSP, as well as sclerosing/sclerotic DFSP, DFSP/DFSP-FS with foci of myoid/myofibroblastic differentiation, DFSP with areas of giant cell fibroblastoma, and DFSP-FS. However, there is only one high-grade variant: DFSP-FS [4], which was first described by Penner [4] in 1951 as a type of metastasizing DFSP with contents histologically indistinguishable from those of fibrosarcoma. DFSP-FS has been reported in 10% to 20% of all DFSP cases [4,5]. DFSP patients usually have a favorable prognosis following surgical resection [4-6], and regular follow-up examinations should be conducted at 6-month intervals for the first 3 years and annually thereafter [5,6]. The metastasis rate in patients with DFSP is less than 5%. However, the prognosis for DFSP-FS appears to be worse, with a higher risk of local recurrence, metastasis, and morbidity [4]. At present, the patient has been receiving follow-up for 10 months, and no complications or metastases have been observed. He also reported that he had no problems with daily activities.
The recurrence rates of hernia after abdominal hernia repair range from 8.7% to 32%, and the risk of recurrence is influenced by several factors, including patient characteristics, surgical technique, the use of mesh, and the treating surgeon’s experience [13]. However, in this case, recurrence of the hernia was not observed during 10 months of follow-up.
This study had some limitations. First, a percutaneous biopsy was not performed. The authors of this study and dermatologists empirically diagnosed the mass as angiosarcoma based on clinical features and MRI findings. Given the highly aggressive nature of angiosarcoma and the rapid increase in size over 4 months before the patient’s visit, the authors planned immediate hospitalization and wide excisional biopsy. If a percutaneous biopsy had been performed and the mass had been diagnosed as DFSP-FS, the authors would have considered hernia repair using synthetic or biological mesh and covering the soft tissue defect using a locoregional flap as the first option, as previously stated.
The second limitation relates to the aesthetic and functional outcomes of the skin graft. To date, the graft has been well taken, and no recurrence or complications such as enterocutaneous fistula have been observed in the herniated area that was reinforced with multilayer ADM. However, compared to other possible surgical methods, the functional and aesthetic outcomes of the procedure described in this study were somewhat disappointing. Nonetheless, an advantage of skin grafts is that they allow local recurrence to be detected quickly, and a higher level of surgical methods in the reconstruction ladder can be considered for cases of recurrence and complications.
This case, which involved the reconstruction of a soft tissue defect with an abdominal hernia after tumor resection using a multilayer ADM and a split-thickness graft, may provide valuable insights for surgeons encountering similar situations.