Introduction
Today clinicians frequently encounter complications following radiation exposure due to the increased use of radiation as a treatment and diagnostic modality, including fluoroscopically guided interventions and radiotherapies [1]. Not all radiation exposures are intended. Radiation exposure occasionally occurs due to malpractice and clinicians’ mistakes. With advances in radiology science and technology, the number of sources of radiation has increased, and the chances of being exposed to radiation grew exponentially. Radiation ulcer is a complication that burdens patients both economically and functionally. Radiation induces hypoxia, hypovascularity, and hypocellularity. Moreover, follicular stem cells that help wound healing in response to conventional therapy are greatly injured in the skin adjacent to the radiation ulcer, delaying the healing process. Even after complete recovery, radiation ulcers tend to easily recur with minor trauma [2]. Therefore, radiation ulcers require continuous monitoring and care through a multidisciplinary approach.
CG paste (Daewoong Pharmaceutical, Seoul, Korea) is an injectable form of micronized acellular dermal matrix (ADM) in gelatin suspension and is known to provide a framework that acts as a scaffold for tissue ingrowth and angiogenesis. The authors suggest that injectable ADM could be an alternative novel treatment of choice for radiation ulcers in the case report [3]. We present a case in which a patient with a large radiation ulcer at his coccyx after a fluoroscopically guided intervention recovered completely using injectable ADM and negative pressure wound therapy (NPWT).
The patient provided written informed consent for the publication and use of his images.
Case
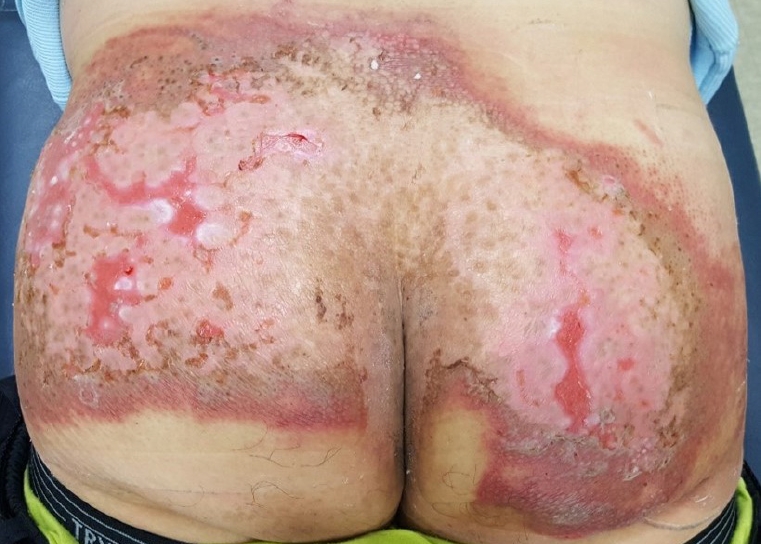
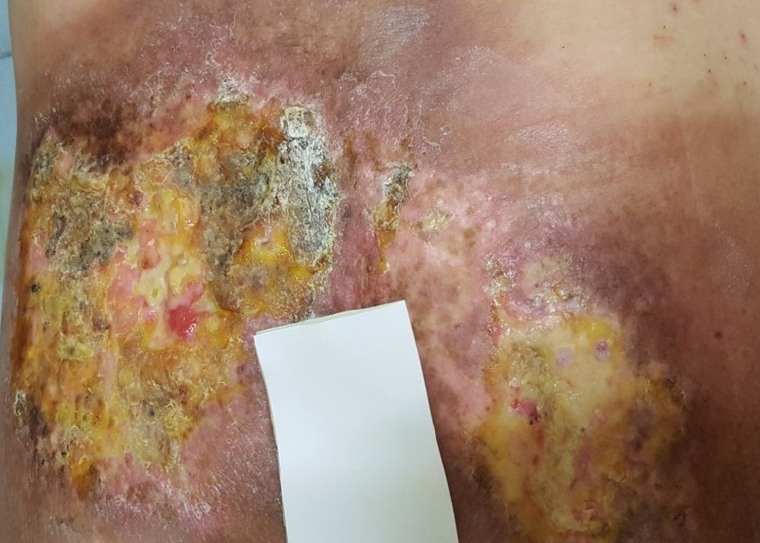
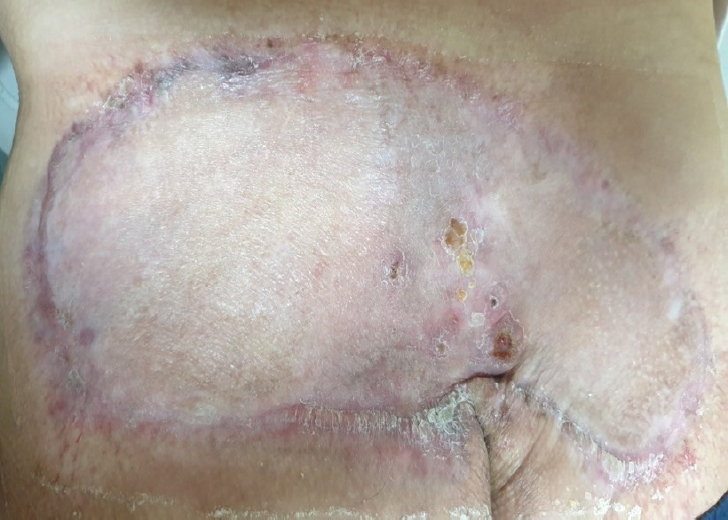
A 49-year-old male patient was referred to our department due to an unhealed 25×10-cm-sized fluoroscopy-induced radiation ulcer located as deep as the muscle layer at his coccyx. The patient was diagnosed with abdominal aortic aneurysm and received embolization for the aneurysm. The procedure took 4 hours, in which fluoroscopy was used for guidance; the patient was naturally exposed to radiation during the entire procedure. The patient reported a mild itching sensation and small bullae at his coccyx on the 10th day after exposure. The skin ulcer aggravated afterward, showing classical signs of radiation ulcer; necrotic changes were observed at the center, the skin adjacent to the ulcer showed sclerotic telangiectasia, and finally, the outermost skin showed pigmentation [2]. The ulcer was treated with a simple dressing and low-intensity laser at the hospital that had performed embolization on the patient before. However, these conventional therapies proved ineffective without any improvement on the patient (Fig. 1). Three months after exposure, the size of the ulcer increased to 25×10 cm with severe fibrotic changes (Fig. 2). The patient visited our clinic 11 months after exposure for further treatment (Fig. 3). Debridement was performed to remove the fibrotic tissue from the ulcer (Fig. 4). NPWT was used for dressing after debridement. The wound was stabilized without any progress 12 weeks postoperatively. Then, 5 mL of injectable ADM were applied twice a week with NPWT for 2 weeks. Granulation tissue was formed on the wound bed 2 weeks after the use of injectable ADM, and the resultant defect was reconstructed with a split-thickness skin graft (Fig. 5). However, a foul-smelling and purulent exudate was detected postoperatively, leading to complete loss of the skin graft. Based on the results of swab cultures obtained from the purulent exudate (Escherichia coli and Pseudomonas aeruginosa), intravenous antibiotic treatment (ertapenem 1 g once a day/ciprofloxacin 400 mg 3 times a day) was administered for 18 days. Simple gauze dressings were changed daily along with 50 mL of normal saline irrigation. The patient was discharged after infection control; conservative therapy through the outpatient clinic was believed to be adequate treatment. A portion of granulation tissue was intact after infection control. Therefore, 2 mL of injectable ADM were applied twice a week for 2 weeks with simple dressing for the remaining defect, which was formed due to loss of granulation tissue. Afterwards, the wound was re-evaluated and the dressing was simplified to simple dressing, as the wound seemed to recover without any complications. After 2 months of conservative dressings, the size of the radiation ulcer did not reduce and maintained a size of 5×3 cm, and our team again applied injectable ADM (1 mL) to the wound on a weekly basis with Mepilex Border (Mölnlycke, Gothenburg, Sweden) dressings for 3 weeks (Fig. 6). Three weeks later, the ulcer healed completely without additional surgery (Fig. 7). In summary, the radiation ulcer, which did not show any improvement for 11 months after radiation exposure, received debridement, conservative dressing utilizing NPWT and injectable ADM, and reconstruction with split-thickness skin graft, which was completely lost due to infection. However, the wound was completely healed by infection control and adequate injectable ADM along with other dressing modalities through constant wound re-evaluation for a duration of nearly 30 weeks.
Discussion
The increasing incidence of fluoroscopy-induced radiation ulcers has become a worldwide trend. However, the disease is often overlooked and underreported due to clinicians’ lack of awareness and patients’ lack of knowledge. Patients administered with fluoroscopy-mediated procedures often lack understanding of the procedures that they are receiving and the possible complications and therefore do not consult clinicians until the disease aggravates and recurs. As a result, demand for treatment of fluoroscopy-induced radiation ulcers is expected to increase, calling for establishment of an effective treatment.
A radiation ulcer is a wound that is hard to heal due to its nature [4]. Ionization causes loss of capillary vessels, which naturally leads to hypoperfusion. Hypoperfusion and hypoxia are known to play key roles in the pathophysiology of numerous hard-to-heal wounds [5]. Inflammatory response, oxidative stress, and genomic etiology also play key roles in radiation ulcers. By understanding these mechanisms of radiation ulcers, clinicians have managed to invent several devices and protocols, such as low-intensity laser and hyperbaric therapy for the management of such conditions [1]. However, even though treatments initially seemed to be promising, none of them proved to be significantly effective and required further studies for clinical application. The patient in our case received a low-intensity laser, a treatment under active research. Studies on such treatments have been conducted in small case series [6]. While in our case, the wound supposedly aggravated due to the low-intensity laser, valid case reports are rare, and references exist on only negligible complications of the modality. Therefore, rather than suspecting low-intensity laser as the culprit, it is more rational to suspect radiation as the cause of the wound aggravation [1].
Surgical reconstruction is another management method for radiation ulcers. Several reports have focused on the importance of radical excision of fibrotic tissues lacking follicular stem cells, which is important in wound recovery [2]. In our case, the skin defect was approximately 25×10 cm in size, which is relatively large when compared to other reported radiation ulcers. Large skin defects require larger flaps associated with a higher risk of flap failure and donor site morbidity [7]. Skin grafts are not a preferable option in radiation ulcers either, as lack of perfusion and tissue destruction due to radiation damage dramatically lowers graft survival rate [8]. Though the patient in our case received a skin graft, the decision was based upon the favorable healing status of the ulcer. Granulation tissue was formed with help of injectable ADM along with NPWT. The authors believed the skin graft could be an option in the case, since granulation tissue showed signs of perfusion. Unfortunately, due to the hypoperfused nature of the radiation ulcer, the skin graft was lost. Chances of infection are higher in hypoperfused tissue, and therefore skin grafts on radiation ulcers are additionally exposed to infection, gradually leading to loss of the graft as in our case.
The authors suggest that injectable ADM can be an alternative novel treatment option for large radiation ulcers. Injectable ADM provides a scaffold for tissue ingrowth and vascular formation. Many studies have utilized ADM as a treatment for various types of ulcers. The use of ADM to salvage radiation ulcers has been reported in various clinical settings, such as breast reconstruction after radiation therapy [9]. Some studies have demonstrated the effective use of ADM for the treatment of diabetic foot ulcers caused by hypoperfusion, similar to radiation ulcers. Increasing evidence indicates that ADM can be used as the treatment for radiation ulcers by facilitating angiogenesis and migration of fibrogenic cells in hypoxic and hypovascular environments [10]. However, treatment of radiation ulcers is still not established; clinicians have yet to propose a promising methodology.
ADM is widely used in various fields of reconstruction and need for the modality is expected to grow in the future due to its efficiency and convenience. Currently sheet type and micronized type of ADM are commercialized, and both forms show great efficacy in reconstruction. As the two forms do not show any difference in efficacy, the micronized type is more preferred in uneven, cavitary form of defects. Even in cases of simple and even defects, the micronized type can be easier to apply due to its flexibility. Because ulcers present to clinicians in various shapes, with many in complex, irregular forms, the micronized type is often preferred for practical reasons. Moreover, the micronized type is ready-to-use and manufactured in a syringe, making it applicable even in outpatient clinics [11]. While patients have difficulty utilizing NPWT as a dressing method due to its burdensome properties, simple dressings are sufficient for injectable ADM, serving well for the purpose of holding ADM in place. Even when patients are supine with regular position changes, simple dressings are sufficient for maintaining adequate contact between injectable ADM and the wound bed.
When manufacturing ADM, cells and cellular components of the dermis are almost completely removed, leaving a mixture of structural and functional proteins behind. Proteins provide the microenvironment for cell adherence, expansion and migration. Collagen, vimentin, laminin, proteoglycan and fibronectin are known proteins that are rich in ADM. Among the components, proteoglycans induce angiogenesis, and laminin maintains binding with connective tissues [12]. Furthermore, ADM is rich in endogenous growth factors such as fibroblast growth factor-2 and transforming growth factor-beta, which facilitate angiogenesis and migration of fibrogenetic cells even in harsh environments such as radiation ulcers [13]. The mechanism works well for radiation ulcers, since key elements of the ulcer are hypocellularity and hypovascularity.
Currently, due to its convenience and efficacy, NPWT is one of the most commonly applied methods of ulcer treatment. It is known to increase dermal perfusion, stimulate granulation tissue formation, and reduce edema and bacterial formation. However, its benefits have been debated, and it is usually used as a management strategy before surgical reconstruction [14]. Numerous studies on ADM combined with NPWT have been conducted; ADM combined with NPWT showed significantly better results than NPWT alone in hypoxic environments similar to radiation ulcers [12]. There has been little discussion on radiation ulcer treatment with NPWT, and in most cases reported, NPWT was utilized only as a modality before surgery to facilitate microcirculation and removal of bacterial proliferation [8,14]. This may be due to the fact that in radiation ulcers, the area of adjacent skin affected by radiation and hence lacking stem cells is usually wider than assumed by clinicians. Therefore, NPWT alone would result in limited management of radiation ulcers [8].
Previous studies using injectable ADM and NPWT proved to be effective in difficult wounds [3]. However, radiation ulcers are different from other wounds in nature. Radiation not only causes vessels to diminish, inducing hypoperfusion and hypoxia, but also prompts necrosis of follicular stem cells, which are the source of recovery. Meanwhile, there are few studies on radiation ulcers, with insufficient research carried out on possible treatments. Therefore, the authors of this study used injectable ADM with NPWT for the radiation ulcer and furthermore explored the possibility of using injectable ADM alone for the radiation ulcer. We suggest injectable ADM for treatment of radiation ulcers and encourage further study.
The article was written as a case report and therefore has many shortcomings. First, since only one case is reported, data on efficacy of ADM on radiation ulcers is limited. A study on a larger scale would be necessary to corroborate our findings and to confirm efficacy of ADM on radiation ulcers. The article aims to suggest the possibility of alternative treatment meriting further study, considering the growing need for better management of radiation ulcers. Second, the patient was treated with ADM combined with NPWT at one point of treatment but was treated with ADM alone at another point of treatment. Therefore, contribution of ADM on recovery of radiation ulcers requires further study.