 |
 |

|
| Aims and Scope |
| About the Journal |
| About the Society (KWMS) |
| Editorial Board |
| Open Access |
| Principles of Transparency and Best Practice |
| Editorial Office |
| Subscriptions |


|
| Aims and Scope |
| About the Journal |
| About the Society (KWMS) |
| Editorial Board |
| Open Access |
| Principles of Transparency and Best Practice |
| Editorial Office |
| Subscriptions |

AbstractBackground Hypertrophic scars and keloids result from burns, trauma, infection, and surgery and affect daily life. Although various scar management options are available, their efficacy remains uncertain. Silicone-based products, including sheets and gels, are the primary choices for scar management. This study assesses the effectiveness of Mepiform and Mepiform Ultra Scar Gel and their optimal use.
Methods Eighteen patients who underwent primary repair between January and June 2021 were enrolled and divided into those using both Mepiform products and those using only Mepiform Ultra Scar Gel. Scars were evaluated at baseline and after 2, 4, 8, 12, and 24 weeks. The Vancouver Scar Scale score was evaluated at 12 and 24 weeks. The patients provided feedback through a survey.
Results Group 1 (both Mepiform products) showed greater improvements in vascularity (33%), height (33%), and overall sum (67%) on the Vancouver Scar Scale from weeks 12 to 24. Group 2 (only Mepiform Ultra Scar Gel) showed improvements in vascularity (22%), height (22%), and overall sum (33%). Both groups reported positive outcomes, with group 1 demonstrating higher improvement percentages for most parameters.
Conclusion This study provides valuable insights into Mepiform and Mepiform Ultra Scar Gel in postoperative scar management despite a limited sample size. All scars in the study either remained stable or improved. Better results from group 1 suggest combining Mepiform products offers advantages. The consistent and prolonged use of silicone-based products is emphasized, and larger-scale research is needed to validate these findings.
IntroductionScarring is a common outcome of injuries and surgical procedures representing the body’s physiological response to tissue repair. Although this natural process is critical for wound closure, excessive or abnormal scarring can lead to functional impairment and profound psychological distress in affected individuals [1]. Hypertrophic scars and keloids stand out among the array of scars and are characterized by raised, erythematous, and often pruritic lesions. Hypertrophic scars are marked by their raised, thickened appearance and typically remain confined to the original wound site [2,3]. They are often reddish or pinkish and may cause itching and discomfort. In contrast, keloids exhibit more aggressive growth, extending beyond the wound boundaries into the surrounding healthy skin. These scars often have a firm and rubbery texture, and hyperpigmentation can make them highly noticeable. Moreover, keloids tend to recur, even after treatment or surgical removal. The underlying mechanism of these scars involves excessive collagen production; however, the distinction lies in the extent and control of collagen overproduction.
The clinical manifestations of hypertrophic scars, keloids, and even linear scars extend beyond the physical level and can significantly affect the quality of life, self-esteem, and psychological well-being of those affected [4]. Patients often experience discomfort, itching, pain, and limitations in their range of motion. Moreover, the conspicuous appearance of these scars can lead to social anxiety and reduced self-confidence, making scar management an essential aspect of comprehensive patient care [5,6].
Over the years, many interventions have been developed for scar prevention and management, including surgical revisions, corticosteroid injections, laser therapy, pressure garments, and topical treatments [7-11]. However, the effectiveness of these strategies varies, and there remains a paucity of consensus on the optimal approach for scar management [7]. Among these interventions, silicone-based products in the form of sheets and gels have emerged as a promising modality with widespread utilization because of their purported ability to improve scar appearance, texture, and symptoms [12-14].
Mepiform and Mepiform Ultra Scar Gel (Mölnlycke Health Care) are notable representatives of silicone-based scar management products. Mepiform is a silicone sheet, and Mepiform Ultra Scar Gel is a clear, viscous gel. These products have garnered attention owing to their ease of use, safety profile, and potential benefits in reducing the visibility and discomfort associated with hypertrophic scars and keloids. Given the prevalence of scarring due to surgical procedures, trauma, burns, and other injuries, there is a need to evaluate the efficacy of silicone-based treatments rigorously. This study investigates the efficacy of Mepiform and Mepiform Ultra Scar Gel in scar management, focusing on comparing their individual and combined use.
MethodsStudy design and participantsThis prospective study enrolled 20 patients who underwent primary repair procedures performed by a senior author between January and June 2021. The study was approved by the Institutional Review Board of Hanyang University Seoul Hospital (IRB No. 2024-02-021), and all patients provided written informed consent before enrollment. Patients were informed that they had the option to withdraw from the study at any point during the research period if they so desired. Patient demographics, including Fitzpatrick skin type, comorbidities, scar details, and surgical history, were collected at the beginning of the study [15]. The inclusion criteria encompassed individuals who had received surgical treatment involving primary closure using sutures, such as scar revision or excisional biopsy. In cases where there were two identical scars that underwent identical surgical procedures, the average values of these scars and results were used for the study. The exclusion criteria included known allergies to silicone-based products and noncompliance with the study protocol. The patients provided written informed consent to use of their collected data and photographs.
Treatment groupsPatients were randomly allocated to one of two distinct treatment groups. This allocation was carried out in an unbiased manner, independent of patient preferences and without direct involvement from the authors. To ensure the randomness of the allocation, a randomization table was utilized. This table was generated and managed by a clinical research coordinator, who was not involved in the direct care of the patients. Each patient was assigned a unique identifier upon entering the study. The research coordinator then used the randomization table to assign each identifier to either group 1 or group 2, thereby determining the patient’s treatment group. This method ensured a transparent and impartial assignment of patients to the respective treatment arms of the study. Group 1 was assigned a combination therapy regimen comprising Mepiform and Mepiform Ultra Scar Gel. The participants in this group were instructed to apply the gel twice a day and use the silicone sheet once the gel had completely dried, with the degree of flexibility allowing them to mimic real-world product usage. In contrast, group 2 received treatment solely with Mepiform Ultra Scar Gel twice a day, allowing for a comparison of the efficacy of these two silicone-based treatments in a real-world clinical setting.
Assessment and follow-upTo comprehensively evaluate the impact of scar management, a multi-dimensional assessment protocol was employed. The patients underwent initial assessments and photography at baseline, followed by subsequent evaluations at 2, 4, 8, 12, and 24 weeks after the initiation of scar management. The baseline was defined as the day of suture removal and the start of scar management. Each assessment involved a detailed examination of the scar characteristics, including vascularity, pigmentation, pliability, height, and overall appearance. These parameters were assessed using the Vancouver Scar Scale (VSS), a validated tool widely used to grade scar severity [16,17]. During the 24-week follow-up period, patients were invited to provide feedback on the products they used through structured surveys.
Statistical analysisHomogeneity tests between the two groups were performed using Mann-Whitney U test, Fisher exact test and chi-square test. Descriptive statistics, including the number of cases, median, interquartile ranges for the VSS scores, were calculated for each group at the 12-week and 24-week assessment periods. The Wilcoxon signed-rank test was applied to assess the statistical significance of changes within each group across the two time points, while the Wilcoxon rank-sum test was employed to evaluate the differences between the two groups at each time point.
ResultsOne patient in group 1, who received a tattoo around the scar during follow-up, and one in group 2, who moved out of the country, were excluded from the study. As a result, 18 patients (nine from each group) were included in the study (Table 1). In group 1 (cases 1–9), all patients were female. In group 2 (cases 10–18), seven patients were female and two were male. The average age of the patients in group 1 was 40 years (range, 25–65 years). The average age of the patients in group 2 was 42.8 years (range, 26–56 years). The patients received different surgical treatments according to their diagnoses. Homogeneity tests for patient characteristics between group 1 and group 2 revealed that all P-values exceeded 0.05, indicating homogeneity between the two groups (Table 2). Photographs of representative results for the two groups are shown in Figs. 1–3.
VSS score resultsGroup 1 (cases 1–9), which utilized the combined therapy approach with Mepiform and Mepiform Ultra Scar Gel, demonstrated notable improvements in VSS score between the 12- and 24-week follow-up assessments (Tables 3, 4). Specifically, vascularity was reduced in 33%, pigmentation improved in 11%, and scar height decreased in 33% of cases in group 1. There was no change in pliability. Overall, 67% of cases showed improvement in the VSS scores. Group 2 (cases 10–18), treated solely with Mepiform Ultra Scar Gel, also had improved scar characteristics over the same 12- to 24-week time frame. Vascularity was reduced in 22%, pliability improved in 22%, and scar height reduced in 28% of cases in group 2. In total, 33% cases of group 2 showed overall improvement in VSS scores. However, no significant pigmentation changes were observed during this period. In groups 1 and 2 combined, vascularity improved in five patients, pigmentation in one patient, pliability in two patients, and height in five patients (Fig. 4).
However, statistical analysis revealed no significant changes within the groups for most variables (Table 5); although there was a notable improvement in the combined sum of VSS scores from 12 to 24 weeks within group 1 (P=0.020). Comparisons between groups did not yield statistically significant differences, indicating similar progressions in scar healing irrespective of the treatment modality. Descriptive statistics for the individual VSS items—Vascularity, Pigmentation, Pliability, and Height—also underscored similar outcomes between both groups throughout the study duration.
Subjective symptom assessment and product surveyIn addition to objective scar assessments, patients were asked if they experienced pruritus or paresthesia, such as burning and tingling sensations in the scars and surrounding skin during each follow-up. We examined key parameters that are frequently mentioned in subjective instruments for scar assessment [17]. Five patients complaining of pruritus and paresthesia reported complete remission during follow-up. This subjective validation aligns with previous research and highlights the significance of patient perspectives in evaluating the success of scar management (Table 6).
Finally, surveys were conducted to evaluate the perceived applicability of both Mepiform and Mepiform Ultra Scar Gel (Tables 7, 8). Patients in group 1, which utilized both Mepiform silicone sheets and Mepiform Ultra Scar Gel, expressed high satisfaction with the usability and performance of Mepiform silicone sheets. They found that the available size met their needs perfectly and that the ease of application was rated very favorably, with all patients finding it easy to apply. Cutting the sheets to fit their scars posed no significant challenges, as indicated by most patients who rated it as “Good” to “Very good.” Moreover, Mepiform showed remarkable conformability to scar contours, adapting well to individual characteristics. The ability to stay in place received varying ratings, from “Poor” to “Very good.” Water resistance during showers received mixed feedback, highlighting the importance of this feature for daily convenience. Patients found Mepiform comfortable to wear, and its thinness was appreciated for discreetness. Ease of removal and the ability to reduce pain during dressing removal were highly rated, contributing to a positive overall impression of Mepiform silicone sheets in scar management for group 1.
Patients in groups 1 and 2 expressed high satisfaction with the usability and performance of the Mepiform Ultra Scar Gel. They found it very easy to apply, and its spreadability was rated “Very good,” making it convenient for covering scar areas evenly. There were some variations in the ratings for drying speed, with overall lower ratings in group 1 than in group 2. The comfort experienced while wearing the gel was consistently high, with patients reporting no significant discomfort or irritation. The thin consistency of the Mepiform Ultra Scar Gel aligned with patient preferences for discreet scar management. The patients in group 2 had a highly positive overall impression of the gel, indicating high satisfaction with its performance and usability. This feedback underscores the user-friendliness and comfort of Mepiform Ultra Scar Gel when used without silicone sheets.
DiscussionManaging hypertrophic scars and keloids remains a significant challenge in clinical practice because patients often experience physical discomfort and psychological distress [18]. Silicone-based products, such as Mepiform and Mepiform Ultra Scar Gel, have emerged as promising modalities for scar management, offering potential benefits in improving scar appearance and reducing associated symptoms. This study investigated the efficacy of silicone-based treatments and the effect of their combined use on scar management.
Despite the limitations of this study, the results provide valuable insights into the effectiveness of Mepiform and Mepiform Ultra Scar Gel in postoperative scar management. This study had some limitations. First, the small sample size and the fact that all patients were of Korean nationality, despite previous studies indicating potential genetic and ethnic factors contributing to keloid and hypertrophic scar formation [19]. The limited sample size made it difficult to draw statistically significant conclusions, and the homogeneity of the patient population raised questions regarding the generalizability of the results to broader demographics. However, to mitigate these limitations, we ensured that the allocation of cases into the two groups was completely randomized.
Second, the absence of a control group is notable. While it may be ethically challenging to have a control group that receives no scar management, the lack of a control group makes it difficult to attribute the observed improvements solely to the use of Mepiform and Mepiform Ultra Scar Gel. However, the efficacy of silicone products in scar improvement has already been demonstrated in several studies, including randomized control tests. Therefore, we can predict that the results derived from this study, as summarized in Fig. 4, would show a better VSS score than if silicone products had not been used [12,13,20]. Moreover, a comparative study with a control group revealed that the group using silicone products exhibited an improvement in VSS scores compared to the control group [13].
The VSS results in both groups 1 and 2 showed overall improvements, although these improvements were not statistically significant. Education on products and usage was emphasized throughout the study because compliance and patient education are crucial in scar management [21]. Group 1, which utilized both Mepiform and Mepiform Ultra Scar Gel, exhibited overall better VSS scores compared to group 2, which used only Mepiform Ultra Scar Gel. “Pressure and Tension Reduction” is a well-established mechanism in scar management [22,23]; silicone sheets, such as Mepiform, provide a physical barrier that gently applies pressure to the scar tissue. This pressure may help flatten raised scars, including hypertrophic scars and keloids, and reduce the risk of abnormal collagen formation [24]. Additionally, it can mitigate the tension on the skin surrounding the scar, which is particularly relevant for scars located in areas prone to high tension or movement [18]. While Mepiform Ultra Scar Gel, with its thinner consistency, may primarily focus on other aspects of scar improvement, such as hydration and modulation of collagen production, it may not exert the same degree of constant pressure as a silicone sheet. Another possible explanation for this difference is the versatility of the two options for scar management. Group 1 had the flexibility to use both the gel and silicone sheet based on their preferences and the characteristics of their scars. This may have led to more consistent and prolonged exposure of scars to silicone-based treatments, potentially contributing to the observed improvements. However, the study was not designed to directly compare the efficacy or strength of Mepiform and Mepiform Ultra Scar Gel, so the relative effectiveness of these products remains unknown.
Regarding the product survey results, it is worth discussing the varying ratings for the “ability to stay in place.” This variation may be attributed to the location of the scars and the characteristics of the skin in these areas. Scars located in areas with hair, such as the eyebrows or skin creases and folds, may present challenges in keeping the product in place compared to scars on flat surfaces, such as the abdomen, inner thighs, and chest walls. Furthermore, transparent gel products may provide better compliance when used in exposed areas such as the face and hands compared to more visible sheet products. In this study, cases 2 and 4 in group 1 required the use of Mepiform sheets on challenging areas such as the eyebrows and earlobes. However, the patients experienced no difficulty in using the product. This was because case 2 had minimal eyebrow hair, and case 4 involved a 1 cm scar located on the flat, anterior surface of the earlobe, allowing for easier adherence of the sheet. Nonetheless, this underscores the importance of considering the anatomical location of scars when selecting products for scar management.
The lower rating of the drying speed of Mepiform Ultra Scar Gel in group 1 may be due to patients having to wait for the gel to completely dry before applying the Mepiform sheet. This additional step in the treatment regimen may have been perceived as a minor inconvenience to patients. However, it is important to note that overall satisfaction with the gel remained high, indicating that this aspect of the treatment did not significantly detract from its usability and effectiveness. By actively incorporating patient feedback and expanding and refining the survey used in this study, it is believed that patient education on scar management and the use of silicone products can be more accurately delivered, leading to superior outcomes.
In conclusion, despite the study’s limitations, it offers valuable insights into the efficacy of Mepiform and Mepiform Ultra Scar Gel in postoperative scar management. These results suggest that combining these products may be advantageous for scar improvement, aligning with the known mechanisms of silicone-based scar management. While numerous studies have investigated the effectiveness of silicone products in scar management, to our knowledge, this is the first study to compare the effects of a combined sheet and gel regimen versus a gel-only regimen. This comparison highlights the potential enhanced benefits of using a combination of products. Further research with larger and more diverse populations is needed to confirm these findings and explore the relative strengths of these products, the established mechanisms of silicone-based scar management support their continued use in clinical practice [14]. This study underscores the importance of the consistent and extended use of silicone-based products in postoperative scar management and the potential benefits they offer to patients seeking to improve both the physical and psychological aspects of their scars. Acknowledging the need for continued investigations, silicone-based scar management remains a valuable option for comprehensive patient care.
Fig. 1.Case 6 in group 1. A 25-year-old female presented with a post-appendectomy hypertrophic scar on her right lower abdomen and received scar revision with multiple Z-plasty. (A) Baseline photograph taken on postoperative day 10 after suture removal. (B) A 4-week follow-up photograph. (C) A 12-week follow-up photograph. The scar received a Vancouver Scar Scale (VSS) score of 3 (vascularity=0, pigmentation=2, pliability=0, height=1). (D) A 24-week follow-up photograph. The scar improved to a VSS score of 2 (vascularity=0, pigmentation=2, pliability=0, height=0). 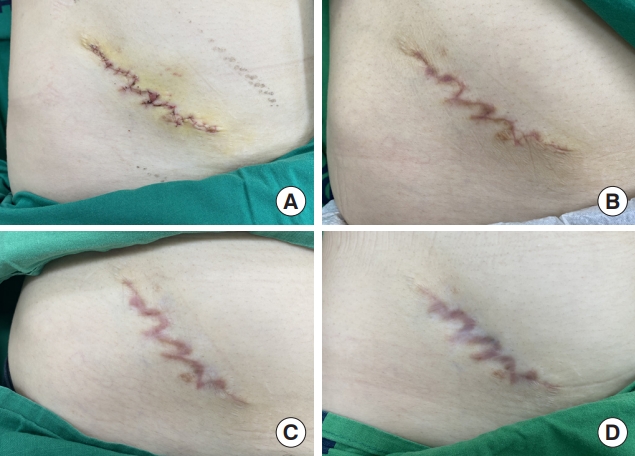
Fig. 2.Case 14 in group 2. A 45-year-old female received revisional rhinoplasty with dermofat graft harvested from the sacral region; primary closure was performed at the donor site. (A) Baseline photograph of the donor site was taken on postoperative day 10 after removal of sutures. (B) A 4-week follow-up photograph. (C) A 12-week follow-up photograph. The scar received a Vancouver Scar Scale (VSS) score of 5 (vascularity=1, pigmentation=2, pliability=1, height=1). (D) A 24-week follow-up photograph. The scar improved to VSS score of 2 (vascularity=0, pigmentation=2, pliability=0, height=0). 
Fig. 3.Case 15 in group 2. A 49-year-old female presented with basal cell carcinoma on her left supramedial cheek and underwent wide excision; the resulting defect was then covered with a local flap. (A) Baseline photograph of the local flap coverage site was taken on postoperative day 7 after removal of sutures. (B) A 4-week follow-up photograph. (C) A 12-week follow-up photograph. The scar received a Vancouver Scar Scale (VSS) score of 3 (vascularity=0, pigmentation=2, pliability=1, height=0). (D) A 24-week follow-up photograph. The VSS score remained constant from 3 to 3. Although there was no improvement in scores from weeks 12 to 24, the scar appearance had become more natural when compared to earlier photographs. 
Fig. 4.Summary of Vancouver Scar Scale (VSS) score improvement. The graph shows percentage of cases that showed improvement of VSS score in groups 1 and 2, and both groups combined. In total, vascularity improved in five cases, pigmentation in one, pliability in two, and height in five. Group 1 was assigned combination therapy consisting of Mepiform (M) and Mepiform Ultra Scar Gel (G) and group 2 was treated with Mepiform Ultra Scar Gel (G) alone twice daily. 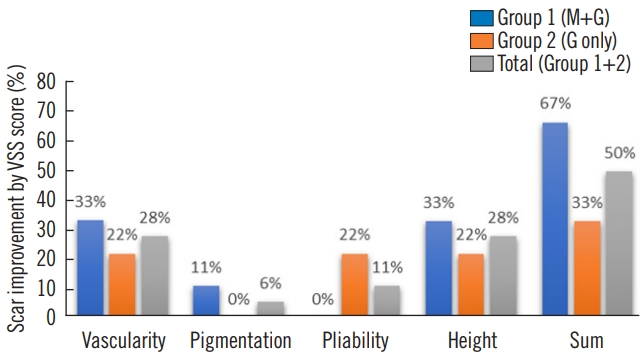
Table 1.Patient characteristics M, Mepiform; G, Mepiform Ultra Scar Gel; DM, diabetes mellitus; ESRD, end-stage renal disease; Rt., right; Lt., left; IQR, interquartile range. Group 1 (cases 1–9): age, median 44 years (IQR, 30–46 years); scar length, median 5.8 cm (IQR, 1.5–8.0 cm). Group 2 (cases 10–18): age, median 47 years (IQR, 30–52 years); scar length, median 4.3 cm (IQR, 2.0–5.0 cm). Table 2.Homogeneity tests between group 1 and group 2 Table 3.Vancouver Scar Scale scar characteristic and score Table 4.Detailed Vancouver Scar Scale score results Table 5.Statistical analysis of Vancouver Scar Scale score results
Table 6.Summary of scar assessments and improvements
Table 7.Overall evaluation of Mepiform Table 8.Overall evaluation of Mepiform Ultra Scar Gel References1. Shen W, Chen L, Tian F. Research progress of scar repair and its influence on physical and mental health. Int J Burns Trauma 2021;11:442-6.
2. Grabowski G, Pacana MJ, Chen E. Keloid and hypertrophic scar formation, prevention, and management: standard review of abnormal scarring in orthopaedic surgery. J Am Acad Orthop Surg 2020;28:e408-14.
3. Ogawa R, Dohi T, Tosa M, et al. The latest strategy for keloid and hypertrophic scar prevention and treatment: the Nippon Medical School (NMS) protocol. J Nippon Med Sch 2021;88:2-9.
4. Lee HJ, Jang YJ. Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. Int J Mol Sci 2018;19:711.
5. Hawash AA, Ingrasci G, Nouri K, et al. Pruritus in keloid scars: mechanisms and treatments. Acta Derm Venereol 2021;101:adv00582.
6. Dodd H, Fletchall S, Starnes C, et al. Current concepts burn rehabilitation, part II: long-term recovery. Clin Plast Surg 2017;44:713-28.
7. Deflorin C, Hohenauer E, Stoop R, et al. Physical management of scar tissue: a systematic review and meta-analysis. J Altern Complement Med 2020;26:854-65.
8. Kadakia S, Ducic Y, Jategaonkar A, et al. Scar revision: surgical and nonsurgical options. Facial Plast Surg 2017;33:621-6.
9. Lee Peng G, Kerolus JL. Management of surgical scars. Facial Plast Surg Clin North Am 2019;27:513-7.
10. Shin TM, Bordeaux JS. The role of massage in scar management: a literature review. Dermatol Surg 2012;38:414-23.
11. Zhang DD, Zhao WY, Fang QQ, et al. The efficacy of fractional CO2 laser in acne scar treatment: a meta-analysis. Dermatol Ther 2021;34:e14539.
12. Hsu KC, Luan CW, Tsai YW. Review of silicone gel sheeting and silicone gel for the prevention of hypertrophic scars and keloids. Wounds 2017;29:154-8.
13. Kim JS, Hong JP, Choi JW, et al. The efficacy of a silicone sheet in postoperative scar management. Adv Skin Wound Care 2016;29:414-20.
14. O’Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev 2013;2013:CD003826.
16. Sullivan T, Smith J, Kermode J, et al. Rating the burn scar. J Burn Care Rehabil 1990;11:256-60.
17. da Costa PT, Echevarria-Guanilo ME, Goncalves N, et al. Subjective tools for burn scar assessment: an integrative review. Adv Skin Wound Care 2021;34:1-10.
18. Son D, Harijan A. Overview of surgical scar prevention and management. J Korean Med Sci 2014;29:751-7.
19. Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci 2017;18:606.
20. Fabbrocini G, Marasca C, Ammad S, et al. Assessment of the combined efficacy of needling and the use of silicone gel in the treatment of C-section and other surgical hypertrophic scars and keloids. Adv Skin Wound Care 2016;29:408-11.
22. Khansa I, Harrison B, Janis JE. Evidence-based scar management: how to improve results with technique and technology. Plast Reconstr Surg 2016;138(3 Suppl):165S-178, S.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||