 |
 |

|
| Aims and Scope |
| About the Journal |
| About the Society (KWMS) |
| Editorial Board |
| Open Access |
| Principles of Transparency and Best Practice |
| Editorial Office |
| Subscriptions |


|
| Aims and Scope |
| About the Journal |
| About the Society (KWMS) |
| Editorial Board |
| Open Access |
| Principles of Transparency and Best Practice |
| Editorial Office |
| Subscriptions |

AbstractBackground Acellular dermal matrices (ADMs) have shown promise in promoting tissue regeneration; however, their integration in challenging cases with limited vertical-axis neovascularization remains difficult. This study investigates whether clinically meaningful transverse-axis neovascularization is identified in ADM engraftment and whether this can be further accelerated by platelet-rich plasma (PRP).
Methods ADM and PRP-soaked ADM were implanted into New Zealand rabbits, and histological analysis was performed at different periods to compare neovascularization.
Results Histological analysis revealed that among 32 biopsy sites, there was transverse-axis neovascularization with an average length of 606.89 μm. When divided into two groups for assessing the impact of PRP on transverse-axis neovascularization, the extent of such neovascularization was measured as 582.99 μm in the control group and 630.79 μm in the experimental group. However, there was no statistically significant difference between the two groups (P=0.693).
IntroductionAcellular dermal matrices (ADMs) have been developed to treat various conditions, using an intact dermal scaffold to replace the native extracellular matrix and promote regeneration of host tissues [1,2]. Owing to their superior efficacy compared to synthetic soft tissues, ADMs were first used for the reconstruction of burn wounds in the 1990s. They have since been used in various reconstructions, including burn injury reconstruction, abdominal wall repair, tympanic membrane replacement, dural repair, and gingival grafting [3-5]. ADMs are typically created by freeze-drying a basement membrane obtained from donor skin, which can be of either human or porcine origin. Subsequently, the dermal matrix undergoes a “decellularization process,” using detergent agents to remove all immunogenic materials, including sweat glands, fibroblasts, smooth muscle, and vascular endothelium. This process is essential to render the graft suitable for use in various clinical applications [6,7].
The integration of ADMs in tissue regeneration is significantly challenged by the need for effective neovascularization, particularly in wounds with bone, ligament, or metal hardware exposure. In these clinical scenarios, ADMs may not integrate stably under these conditions where vertical-axis neovascularization is typically restricted due to the underlying avascular structures, forcing reliance on more limited transverse-axis vascular development [8,9]. Therefore, the importance of adjuvant strategies involving mesenchymal stem cells, platelet-rich plasma (PRP), and modified ADMs to enhance neovascularization cannot be overlooked [3,5,9-11].
Recent studies have demonstrated the potential of PRP to enhance wound healing and tissue regeneration [10,12,13]. Specifically, PRP plays a pivotal role in promoting the engraftment and integration of ADMs used for trauma and burn wound reconstruction. This regenerative capacity is attributed to the myriad of mitogenic and chemotactic growth factors released by platelet concentrates. When activated, these factors not only help achieve hemostasis via fibrin clot formation, but also provide a bioactive scaffold matrix facilitating migration of regenerative tissue-forming cells and endothelial involved in vascularization and tissue remodeling.
On the basis of these established facts, this study aims to evaluate the clinical significance of transverse-axis neovascularization in ADM engraftment under conditions where vertical-axis neovascularization is limited. Additionally, it seeks to ascertain whether the application of PRP significantly enhances neovascularization.
MethodsAnimal modelFour adult male New Zealand rabbits (Oryctolagus cuniculus) with a mean weight of 4.0 kg (range, 3.6–4.3 kg) were used in the experiment. Randomization was performed using the online resource random.org. Each rabbit was housed individually in a separate cage at a controlled temperature of 18‒20 oC and fed a standard pellet diet (Oxbow Garden Select pellets; Oxbow Animal Health). All animal procedures and care were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC No. #KMAP22-03) and performed in accordance with all Association for Assessment and Accreditation of Laboratory Animal Care International and IACUC standards.
PRP isolationPreoperatively, 10 mL of blood was collected from each rabbit’s ear into a vacuum flask containing 2 mL of the anticoagulant citrate dextrose solution formula A. The collected blood was then centrifuged at 180×g for 10 minutes at room temperature. The supernatant, including the buffy coat, was subsequently separated from the lower layer of red blood cells. Then a second centrifugation step was performed at 500×g for 10 minutes to remove the plasma floating in the upper layer. The remaining concentrated platelet solution contained an average platelet count of 7.5×105 cells.
Surgical procedureAll surgical procedures were performed under sterile conditions. General anesthesia was induced by intramuscular injection of ketamine (0.25 mL/kg) and xylazine (0.05 mL/kg), while anesthesia was maintained through inhalation of isoflurane via a face mask. Skin incisions were made on the back fascia over the trapezius and latissimus muscles of each experimental rabbit, creating a total of eight pocket spaces per rabbit. Intact ADM (Strattice; Allegheny Technologies), 1.5–2.0 mm thick, was precisely cut into rectangular shapes measuring 2×1.2 cm, and the top and bottom of the porous cross sections were carefully wrapped with silicone to prevent vertical-axis neovascularization. Prior to implantation, the ADM rectangles designated for PRP treatment were immersed in 200 μL of PRP, which was concentrated at 7.5×105 platelets per μL in plasma for 5 minutes to ensure thorough saturation [14]. These silicone-wrapped ADMs were then inserted into the subfascial spaces (Figs. 1, 2). Four pocket spaces were implanted with unsoaked ADM, while the remaining four sites were implanted with ADM soaked in PRP (Fig. 1). All implants were fixed with a 5-0 round needle PROLENE suture. One pure ADM and one PRP-soaked ADM were harvested from each rabbit at weeks 1, 2, 3, and 4 (four pairs per week). The surgical sites were monitored for infections and other complications throughout the recovery period.
Histological analysisTo ensure accurate measurements, the ADM abutting the fascia was removed prior to histological analysis, and only the portion surrounded by silicone was used for examination. The prepared specimens intended for histologic analysis were rapidly excised and fixed in 10% formalin for paraffin sectioning and then sectioned into 4-μm-thick slices for hematoxylin and eosin (H&E) staining. Light microscopy was used to identify vascular endothelial cells formed within the dermal matrix and to analyze the extent of transverse-axis neovascularization. ImageJ software version 1.53t (National Institutes of Health) was used to calculate these measurements. All histological measurements were performed by two independent observers who were blinded to the experimental conditions.
Statistical analysesDescriptive statistical analysis was conducted in the control group to determine whether transverse-axis neovascularization occurs in the absence of PRP. Given the non-normal distribution of the experimental values, a Mann-Whitney U test was used to compare the control and experimental groups. Additionally, a repeated measures analysis of variance (ANOVA test) was performed to assess potential differences between the groups at each weekly time point. All statistical analyses were performed using IBM SPSS software version 28.0 (IBM Corporation). Statistical significance was set at P<0.05.
ResultsMacroscopic findingsAll animals in the experiment survived and remained in good health. None of the rabbits showed any adverse reactions to ADM or silicone. All stitches were removed on postoperative day 7 following implantation or sampling.
Histological analysisThe results of H&E staining indicated that in both the control and ADM+PRP groups, the implanted ADM retained its original structure, with the presence of surrounding inflammatory cells. Neovascularization was observed in both groups (Fig. 3). Histological analysis of the two groups revealed no microscopic differences in inflammatory cell activity or the number of fibroblasts.
Transverse-axis neovascularization in the control group (ADM only)The extent of transverse-axis neovascularization within the dermal matrix was quantitatively assessed by measuring the distance from the margin to the continuously generated vascular endothelial cells (Fig. 4). Throughout the 4-week experimental observation, transverse-axis neovascularization was observed even in the untreated ADM group (Table 1). However, attributing clinical significance to these findings is challenging when considering the weekly variation and the specific measurements. Moreover, the extent of neovascularization, although present, did not demonstrate a consistent trend over time, suggesting limited clinical relevance in the untreated ADM scenario.
A comparative study of ADM vs. ADM with PRPA Mann-Whitney U test was conducted to evaluate the statistical significance of the differences in neovascularization between the control and PRP groups, revealing no significant differences (P=0.158) (Table 2). This result suggests that the application of PRP might not significantly enhance the extent of transverse-axis neovascularization in ADMs.
Repeated measures ANOVA test analysisIn the analysis using repeated measures ANOVA, the impact of PRP treatment on the extent of neovascularization was assessed across all measured time points (Fig. 3). The results indicated that the application of PRP did not significantly influence the degree of neovascularization at any time point throughout the study period (P=0.693). Furthermore, post-hoc analysis using Tukey’s honest significant difference test revealed no significant pairwise differences between the groups at any time point (P>0.05). These results suggest that the extent of neovascularization did not vary significantly between the control and PRP groups throughout the study and did not exhibit significant changes over time, irrespective of the treatment.
DiscussionThe present study aimed to determine the clinical implications of transverse-axis neovascularization in scenarios where conventional vertical-axis neovascularization faces constraints, a common challenge in clinical contexts. Furthermore, recognizing the angiogenic potential of PRP, this study explored the clinical significance of transverse-axis neovascularization and assessed the efficacy of PRP in facilitating ADM engraftment.
Extensive bodies of previous research have focused on the healing mechanism and effectiveness of autologous PRP. While these studies have extensively investigated the regenerative properties of PRP in skin-related applications, including dermatology and aesthetic medicine, there is a scarcity of research specifically focusing on its use in combination with ADM [10,12]. Notably, Fareid et al. [5] conducted a retrospective study involving 14 patients to demonstrate the efficacy of ADM allografts with PRP in the management of gingival recession and demonstrated that soaking ADM grafts in PRP is a valuable and reliable surgical technique.
To provide a comprehensive understanding of these findings, it is essential to situate them within the broader scope of existing research on neovascularization. Neovascularization, fundamentally, involves the formation of new blood vessels from preexisting vasculature. This process is critical in various physiological and pathological conditions, including wound healing, ischemic diseases, and tumor growth. Studies have shown that specific growth factors such as vascular endothelial growth factor and platelet-derived growth factor, which are abundant in PRP, play a pivotal role in stimulating this process [15]. These growth factors promote endothelial cell proliferation and migration, leading to the formation of new capillaries.
The use of H&E staining as our primary technique for studying neovascularization is grounded in its proven effectiveness and reliability in histological studies. H&E staining allows for the clear visualization of tissue structure and morphology, which is crucial in identifying and quantifying new blood vessel formation [16,17]. While it is acknowledged that H&E staining might not provide the functional specificity of techniques like immunohistochemistry, its simplicity and widespread availability make it a valuable tool in preliminary investigations of neovascularization. Moreover, numerous studies have successfully utilized H&E staining to quantify neovascularization, reinforcing its validity in our research approach.
In the present study, the experimental measurements showed that even in the control group, which received no treatment, the mean extent of transverse-axis neovascularization was 582.99 μm within the 4-week study duration, promoting questions about the clinical meaningfulness of such neovascularization. Specifically, while the experimental group exhibited a trend towards enhanced vascularization compared to the control group, the differences did not reach statistical significance (P=0.693) (Fig. 5). This unexpected outcome challenges the established angiogenic profile of PRP and highlights the complexity of ADM engraftment and the multifactorial nature of neovascularization. Additionally, while the experimental model did not reveal a substantial influence of PRP on transverse-axis neovascularization, it highlights the ongoing need for refinement and exploration in this field. Future studies focusing on long-term effects, various bioactive agents, and different ADM types are crucial in deepening our understanding of ADM engraftment strategies.
It is also important to note that the present study had several limitations. The limited number of animals in our model may limit the generalizability of our findings. Additionally, potential biases during sample collection, such as section damage, may have impacted the reliability of the results. The 4-week timeframe of the study also poses a limitation, as it may not have been adequate to observe the long-term effects of PRP on ADM engraftment, and an extended duration might reveal more pronounced effects of transverse-axis neovascularization. Hence, the clinical relevance of an extended experimental duration remains uncertain, given the practical challenges in maintaining ADM grafts beyond this period. Future investigations should explore larger samples and longer study durations to better understand the long-term effects of PRP on ADM engraftment. Further, comparative studies involving other bioactive agents, such as adipose-derived stem cells, and different types of ADMs could further elucidate strategies for optimizing ADM integration. Moreover, implementing additional staining methods, such as immunohistochemistry, would provide deeper insights into neovascularization dynamics, enhancing the comprehensiveness of the results of the present study.
Conflict of InterestThis work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1G1A1007678). No other potential conflict of interest relevant to this article was reported. Fig. 1.Transverse-axis neovascularization and acellular dermal matrix (ADM) process overview. (A) Schematic for evaluating transverse-axis neovascularization. (B) Schematic of ADM harvesting schedule. Control, control group (pure ADM); PRP, experimental group (ADM soaked with platelet-rich plasma); W, week. 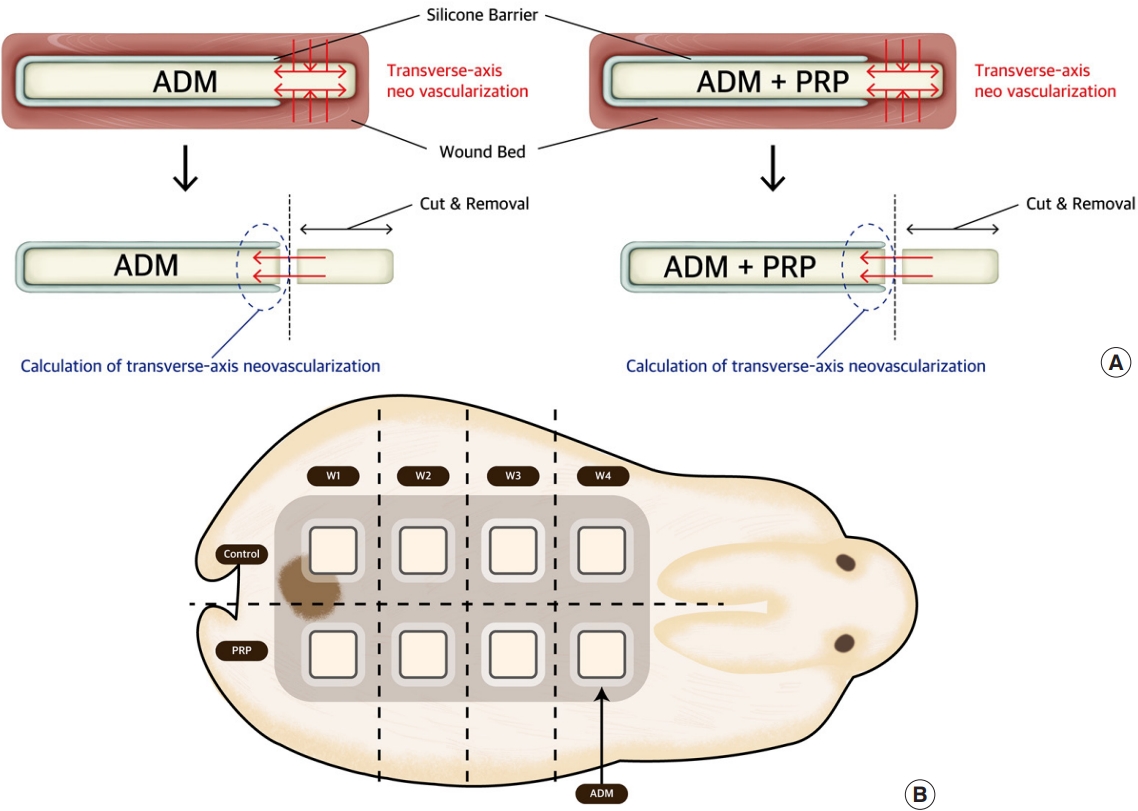
Fig. 2.Clinical photos of the acellular dermal matrix (ADM) implantation process. (A) Silicone barriers were fixated above and below the ADMs. (B) Incisions were made in the back fascia over the trapezius and latissimus muscles. (C) Four sites were implanted with pure ADM, and four sites were implanted with ADM with platelet-rich plasma symmetrically. 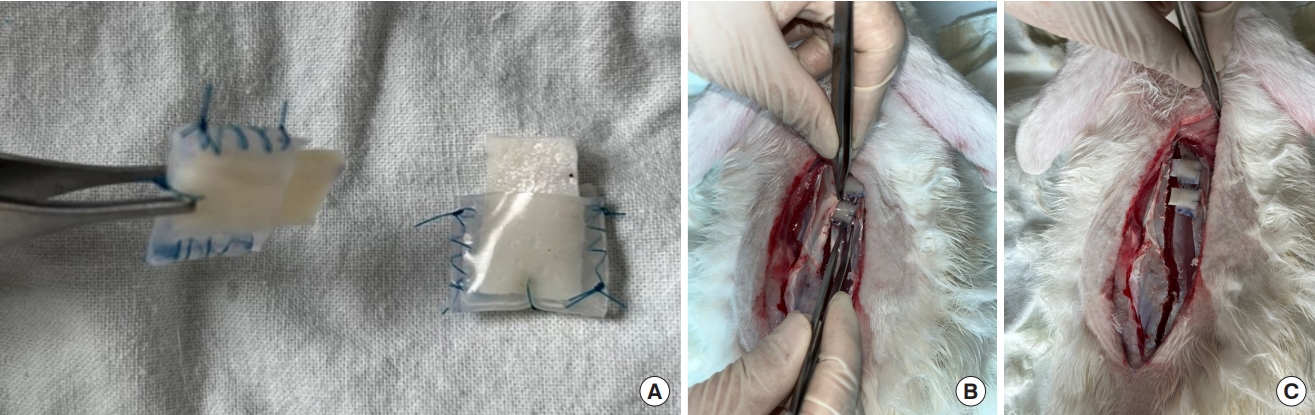
Fig. 3.Histologic analysis through hematoxylin and eosin (H&E) staining. (A, B) H&E staining of a sample from week 3, control group. Few vascular endothelial cells are observed between the pinkish-stained dermal matrix structures. (C, D) H&E staining of a sample from week 3, platelet-rich plasma group. Vascular channels are observed as hyperchromatic endothelial cells (yellow arrows) between pink-stained dermal matrix structures. 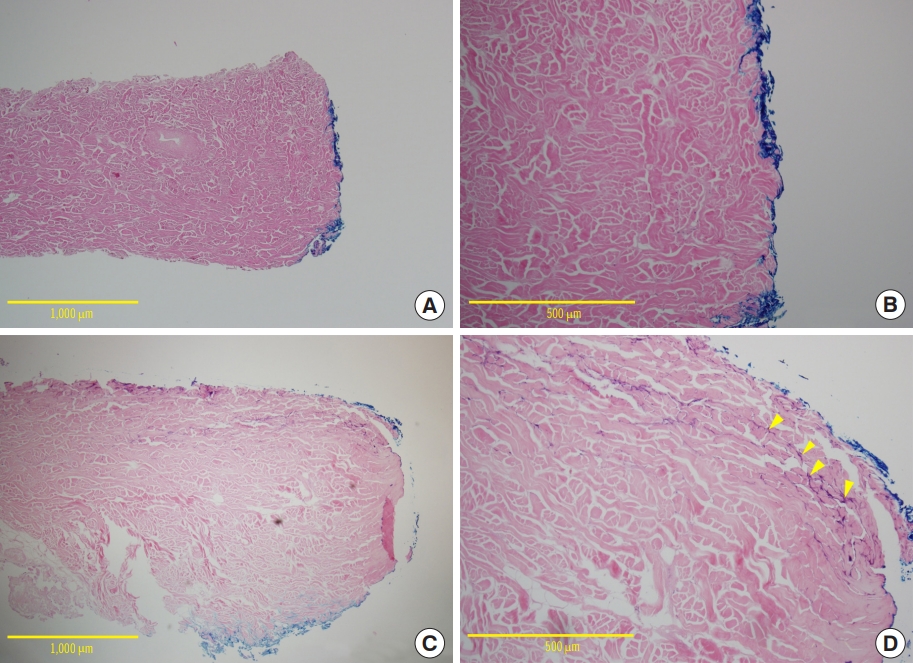
Fig. 4.Calculation of the extent of transverse-axis neovascularization. The sample collected from the platelet-rich plasma group at week 4 of the experiment was subjected to hematoxylin and eosin staining and then assessed under a light microscope using the Image J software, revealing that the extent from the margin to the area where the endothelial cell grew was 669.28 μm. 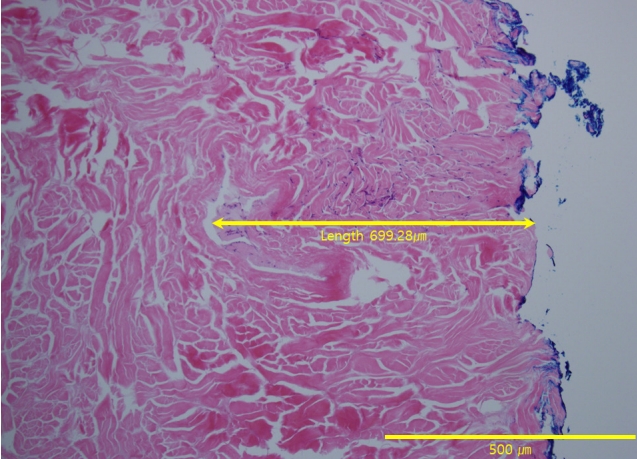
Fig. 5.Profile plot of repeated measures analysis of variance test. Extent of transverse-axis neovascularization. C, control group (pure acellular dermal matrix); P, experimental group (acellular dermal matrix soaked with platelet-rich plasma). 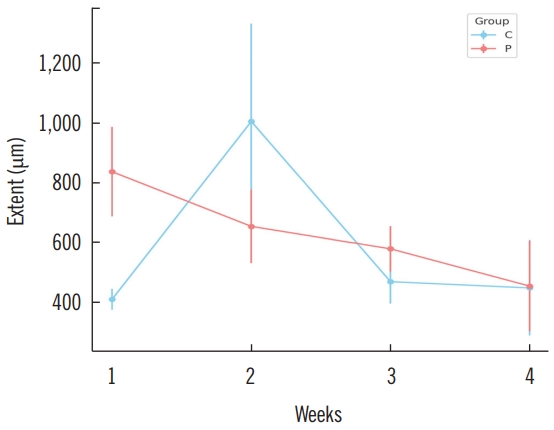
Table 1.Weekly comparison of transverse-axis neovascularization in the control group (ADM only)
Table 2.Comparative analysis of transverse-axis neovascularization: control (ADM only) vs. PRP groups (ADM with PRP)
References1. Petrie K, Cox CT, Becker BC, et al. Clinical applications of acellular dermal matrices: a review. Scars Burn Heal 2022;8:20595131211038313.
2. Fosnot J, Kovach SJ 3rd, Serletti JM. Acellular dermal matrix: general principles for the plastic surgeon. Aesthet Surg J 2011;31(7 Suppl):5S-12, S.
3. Surowiecka A, Chrapusta A, Klimeczek-Chrapusta M, et al. Mesenchymal stem cells in burn wound management. Int J Mol Sci 2022;23:15339.
4. Cetiner D, Gokalp Kalabay P, Ozdemir B, et al. Efficiency of platelet-rich plasma on acellular dermal matrix application with coronally advanced flap in the treatment of multiple adjacent gingival recessions: a randomized controlled clinical trial. J Dent Sci 2018;13:198-206.
5. Fareid R, El-Mofty M, Ghaffar KA. Clinical evaluation of platelet-rich plasma and acellular dermal matrix allograft in the management of gingival recession. Egypt J Oral Maxil lofac Surg 2011;2:81-6.
6. Gierek M, Labus W, Kitala D, et al. Human acellular dermal matrix in reconstructive surgery: a review. Biomedicines 2022;10:2870.
7. Mihalecko J, Bohac M, Danisovic L, et al. Acellular dermal matrix in plastic and reconstructive surgery. Physiol Res 2022;71(Suppl 1):S51-7.
8. Shah A, Taupin P. Strategies for extremity reconstruction with exposed bones and tendons using acellular dermal matrices: concept of sequential vascularization. Case Reports Plast Surg Hand Surg 2021;9:7-14.
9. Kim MJ, Lee WB, Park BY. Effect of morphologically transformed acellular dermal matrix on chronic diabetic wounds: an experimental study in a calvarial bone exposure diabetic rat model. J Surg Res 2022;272:153-65.
10. Gierek M, Klama-Baryla A, Labus W, et al. Platelet-rich plasma and acellular dermal matrix in the surgical treatment of hidradenitis suppurativa: a comparative retrospective study. J Clin Med 2023;12:2112.
11. Kazakos K, Lyras DN, Verettas D, et al. The use of autologous PRP gel as an aid in the management of acute trauma wounds. Injury 2009;40:801-5.
12. Del Pino-Sedeno T, Trujillo-Martin MM, Andia I, et al. Platelet-rich plasma for the treatment of diabetic foot ulcers: a meta-analysis. Wound Repair Regen 2019;27:170-82.
13. Jeong WH, Roh TS, Kim YS, et al. Acceleration of osteogenesis by platelet-rich plasma with acellular dermal matrix in a calvarial defect model. Childs Nerv Syst 2016;32:1653-9.
14. Araujo-Gutierrez R, Van Eps JL, Scherba JC, et al. Platelet rich plasma concentration improves biologic mesh incorporation and decreases multinucleated giant cells in a dose dependent fashion. J Tissue Eng Regen Med 2021;15:1037-46.
15. Kakudo N, Morimoto N, Kushida S, et al. Platelet-rich plasma releasate promotes angiogenesis in vitro and in vivo. Med Mol Morphol 2014;47:83-9.
16. Hamidinekoo A, Kelsey A, Trahearn N, et al. Automated quantification of blood microvessels in hematoxylin and eosin whole slide images. Proc Mach Learn Res 2021;156:94-104.
|
|