Introduction
Exosomes are small extracellular vesicles containing protein, lipid, RNA and DNA, and their diameter is between 30 and 150 nm, originating from multivesicular bodies [1,2]. When absorbed into other cells, exosomes transfer their contents to the cytoplasm of target cells, altering the physiological state of the recipient cells. Exosomes are capable of engaging in intracellular communication as well. Moreover, research has demonstrated that exosomes can function as biomarkers in the diagnosis and prognosis of illnesses [3].
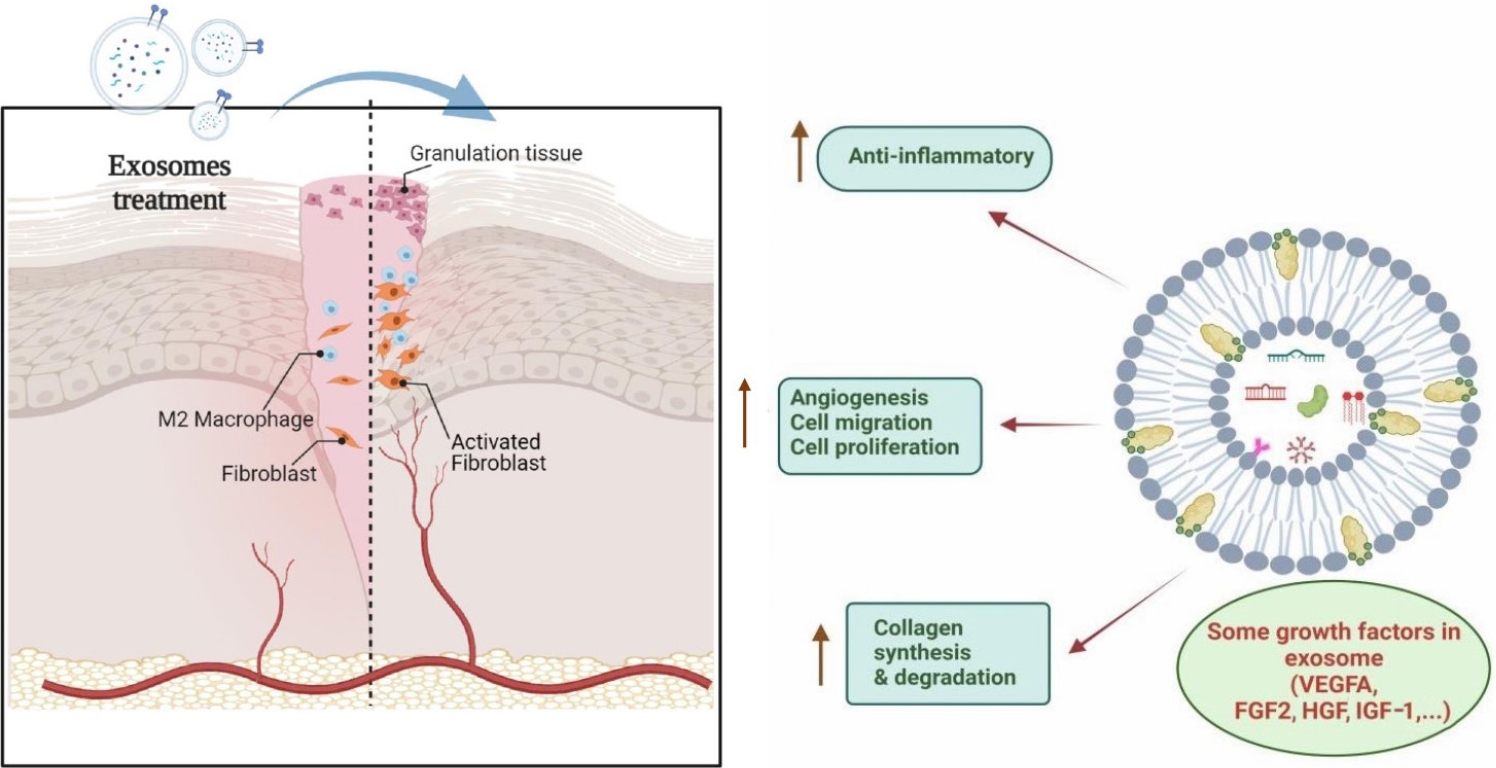
Exosomes are secreted by various cells such as mesenchymal stromal/stem cells (MSCs) [4], immune cells [5], cancerous cells [6], and microglia [7]. Cells that aid in the healing of wounds, like keratinocytes, macrophages, fibroblasts, and endothelial cells can exhibit functional alterations in the wound environment. These alterations impair wound healing by causing prolonged inflammation, poor angiogenesis, ischemia, hypoxia, as well as decreased collagen synthesis. To encourage wound healing, a novel strategy must be developed [8].
Wound healing is generally divided into four main stages: (1) homeostasis; (2) inflammation; (3) proliferation; and finally; and (4) skin regeneration/remodeling, which leads to architectural and physiological restoration after lesion/injury [9]. Exosomes released from various sources have the ability to influence the behavior of cells during wound healing, enhance neovascularization, promote collagen deposition, reduce inflammation, and speed up the healing process (Fig. 1) [10]. Numerous studies have demonstrated that applying these nanoparticles directly to injured tissues can have effects akin to those of utilizing stem cells, without entailing many of the dangers of stem cell transplant therapy [11,12]. Recently, a group of researchers identified a subpopulation of cells obtained from urine that are similar to MSCs in terms of their biological traits, including cell surface markers, multipotent differentiation, proangiogenic effects, self-renewal capacity, and immunomodulatory properties. Taking the name from their origin, these cells are called urine-derived stem cells (USCs) [13].
USCs are preferrable to MSCs for at least four reasons: (1) a person’s health status (apart from urinary tract infections) does not affect the availability of USCs; (2) USCs can be collected from any age, sex, or condition; (3) isolating pure USCs is easy, safe, non-invasive, and inexpensive; and (4) USCs have high telomerase activity to produce more cells, but they do not form teratomas or tumors [14,15].
The aforementioned factors render USCs a desirable cell source for exosome production. According to a study, local USC transplantation greatly accelerated wound healing and angiogenesis in an animal model [16]. One of the key important proteins that is highly expressed in exosomes made from urine stem cells is deleted in malignant brain tumors 1 (DMBT1). DMBT1, a member of the scavenger receptor cysteine-rich (SRCR) protein superfamily, plays a role in inflammation, innate immune activation, cell differentiation, and polarity [17].
Previous research indicates that DMBT1 can act as an intestinal mucosal integrity mediator, Golgi cargo receptor in the context of regulated secretion, regulator of epithelial differentiation, and suppressor of bacterial pathogens [18]. Further evidence suggests that DMBT1 actively stimulates endothelial cell adhesion, migration, proliferation, vascular repair, and angiogenesis under ischemic conditions [19].
Not many studies have delved into the role of DMBT1 in wound healing. This review focuses on the role of the exosomal protein, with an emphasis on its impact in wound healing.
Deleted in malignant brain tumors 1
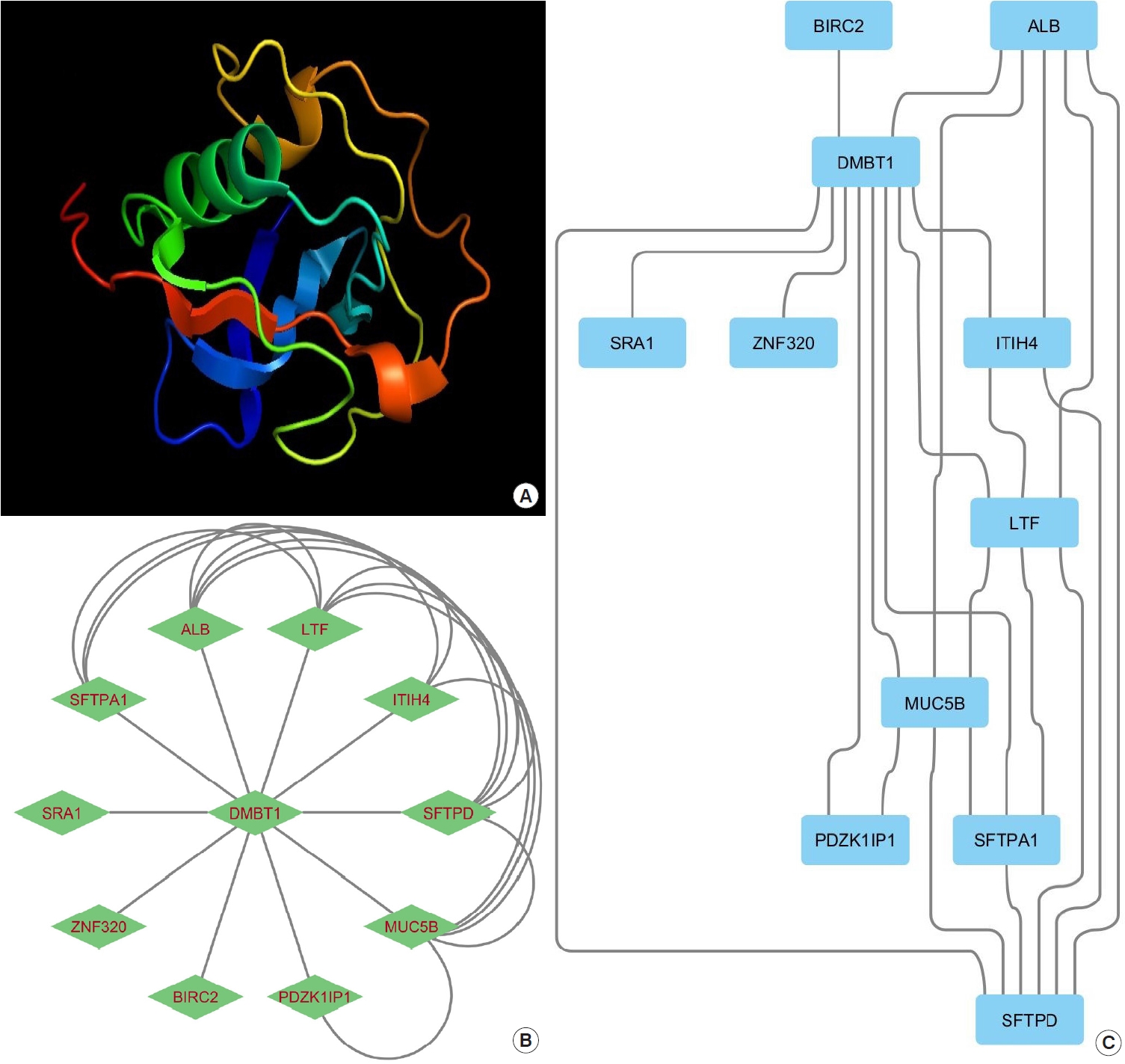
A medulloblastoma cell line that had a homozygously deleted sequence in the region between markers D10S209 and D10S587 distal to 10q was reported by Mollenhauer et al. [20]. The authors discovered a gene spanning the whole candidate region in genomic clone contigs encompassing the deleted region, which they named DMBT1 (Fig. 2). This predicted polypeptide exhibits homology with the SRCR superfamily of SRCR receptors [20]. Mollenhauer et al. discovered for the first time that two out of 20 medulloblastomas and nine out of 39 glioblastomas multiforme (GBMs) had intragenic homozygous deletions in DMBT1. DMBT1 was not expressed by four of the five cell lines from brain tumors. Moellenhauer et al. [20] speculated that DMBT1 functions as a tumor suppressor and may be involved in the carcinogenesis of medulloblastoma and GBM.
A patient with alveolar proteinosis had his human bronchi cleaned by Holmskov et al. of a glycoprotein (GP-340). GP-340 was discovered because it bound to lung surfactant protein D in a calcium-dependent manner (SFTPD) (Fig. 2) [21].
The lung, trachea, salivary glands, small intestine, and stomach were found to be the primary expression sites of GP-340, according to reverse transcription polymerase chain reaction. The distribution of GP340 in macrophages aligned with its role as an SFTPD opsonin receptor [22].
Researchers discovered that there is a protein-protein interaction between SFTPD and GP340, and that SFTPD’s carbohydrate recognition domain mediates the connection to GP340 [21]. It was found by Holmskov et al. [21] that GP340 is present in alveolar macrophage membranes and in a soluble form. The nucleotide sequences allowed the researchers to show that GP340 and the 6.1-kb variant of DMBT1 are different splice variants of the same gene.
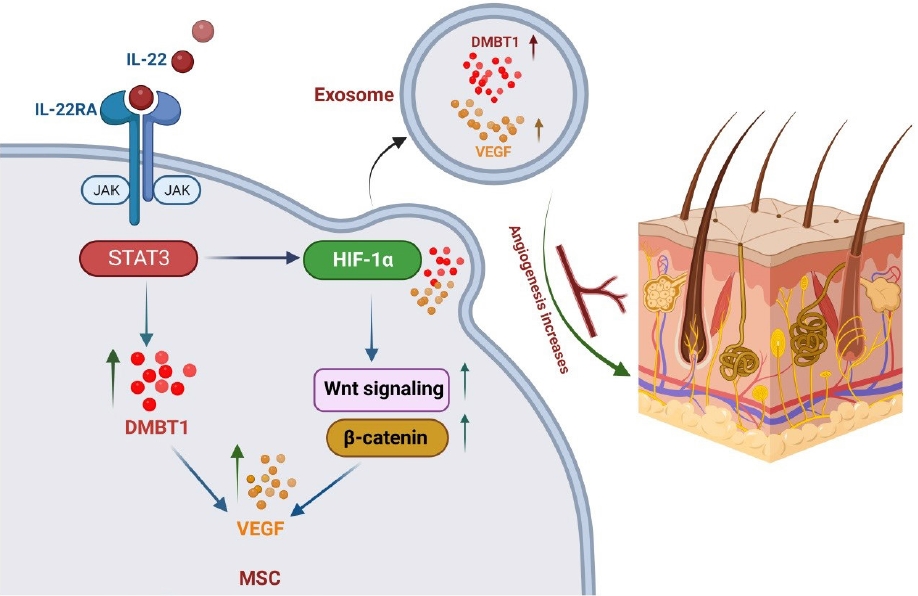
USCs were positive for CD73, CD44, CD29, and CD90; however, CD45 and CD34 were negative. Osteoblasts, adipocytes, and chondrocytes can all be differentiated from USCs. The spherical-shaped USC-exosomes, which are stem cell exosomes derived from human urine, had a mean diameter of 51.57±2.93 nm. TSG101 and CD63 also had contained positive expressions. There was an abundance of proteins in USCs-exosomes that regulate biological processes associated with wound healing. Specifically, the pro-angiogenic protein DMBT1 was highly expressed in USCs-exosomes (Fig. 3) [19].
The secreted epithelial glycoprotein DMBT1, also referred to as salivary agglutinin, muclin, hensin, surfactant, pulmonary-associated protein D-binding protein, and GP-340, is expressed in various bodily tissues, including the liver, kidney, epidermis, salivary glands, and lungs [23]. Tumor suppressor [24], regulator of epithelial differentiation [17], Golgi cargo receptor in the regulated secretory pathway [25], part of the innate immune defense against bacterial pathogens [26], and intestinal mucosa protector [18] are the most studied of the many proposed functions of DMBT1 protein. According to recent research, DMBT1 promotes endothelial cell adhesion, migration, and proliferation in mouse models of limb ischemia, which contributes to angiogenesis [27]. Additionally, by increasing the production of vascular endothelial growth factor A (VEGF-A) and lowering the production of interleukin (IL)-6, DMBT1 can cause angiogenic responses in alveolar tissues (i.e., in type II lung epithelial cells) [28].
Studies have demonstrated that VEGF-A and PI3K-Akt axis are DMBT1’s downstream targets [28,29]. A different study reports that DMBT1-enriched USC-exosomes can significantly increase Akt phosphorylation and VEGF-A protein levels. In actuality, DMBT1 is crucial because it acts as a mediator in the control of angiogenesis and wound healing caused by USC-exosomes. At the same time, it should be highlighted that DMBT1 inhibition did not totally eliminate the effects of USC-exosome on angiogenesis and wound healing in vitro and in vivo, indicating the possibility of additional signaling molecules and pathways being involved in these processes [19].
Researchers have found that in rats with diabetic ulcers, the oxygen-releasing dressing OxOBand, which is derived from adipose-derived MSCs (ADSC), can speed up wound closure, re-epithelialization, and the formation of granulation tissue, while also alleviating infectious wound healing [30]. In another study, ADSC-exosomes were used to stimulate human umbilical vein endothelial cell tubular formation in vitro as well as the migration and proliferation of human dermal fibroblasts. However, ADSC-exosomes can be transported to diabetic ulcers using a naturally occurring acellular scaffold called human acellular amniotic membrane scaffold dressing. This accelerates wound healing in the animal model by decreasing the inflammatory response, promoting angiogenesis, and increasing extracellular matrix accumulation in diabetic mice [31]. Overall, these investigations demonstrated the remarkable potential of exosomes in the healing of wounds, including diabetic wounds.
Other influential factors in small extracellular vesicles that accelerate wound healing
Hemostasis
Research has shown that the primary therapeutic benefit of platelets during hemorrhage depends on the extracellular vesicles that properly functioning platelets produce [32]. Exosomal plasma can enrich many proteins involved in hemostasis, including integrins αIIb and β3, filaggrin-A, platelet chemotactic protein (PF4), as well as CD36 [33]. Platelet exosomal can improve trauma results by retaining hemodynamic stability and decreasing the development of metabolic acidosis and ischemia.
Inflammation
A complicated biological reaction to damaging stimuli like infections and damaged cells is inflammation. Blood arteries, immune cells, and molecular bridges are all part of this defense mechanism. In actuality, inflammation serves as a protective mechanism for wounds [34]. MSCs-derived exosomes can down-regulate pro-inflammatory factors, such as cyclooxygenase (COX2) and inducible nitric oxide synthase, chemokines and cytokines [35].
Moreover, MSC-derived exosomes have the ability to upregulate the anti-inflammatory cytokine IL-10, which has been shown to be crucial in regulating skin wound inflammation [36]. Prominent inflammatory cells called macrophages are essential to the skin’s regeneration process. The transition of macrophages to the M2 phenotype—an anti-inflammatory macrophage— can be accelerated by MSC-derived exosomes [37].
Furthermore, the JAK2/STAT6 signaling pathway is required by MSC-derived exosomes to mediate macrophage activation, which can dramatically lower the quantity of pro-inflammatory macrophages [38]. By controlling phosphatase and tensin homolog (PTEN) and blocking Akt phosphorylation, melatonin-pretreated MSC-derived exosomes can strengthen the M2 phenotype and effectively inhibit the production of IL-1β and tumor necrosis factor-α. Additionally, it raises the level of IL-10 expression [39].
Studies have shown that MSC-derived small extracellular vesicles can convert activated T lymphocytes to the regulatory T cell, thus creating an immunosuppressive effect [40]. Additionally, research has shown that particular microRNAs (miRNAs) allow nanosized extracellular vesicles derived from MSCs to modulate immune responses. For example, specific regulation of inflammation has been demonstrated by miR-21, miR-146a, and miR-181c that are enriched in extracellular vesicles derived from human umbilical cord-MSCs [41].
All things considered, more investigation is required to clarify the distinct molecular pathways underlying MSC-derived nanovesicles’ ability to inhibit inflammation during wound healing.
Angiogenesis
A previous study demonstrated the presence of numerous angiogenic factors in MSC-derived exosomes, including thrombopoietin, fibroblast growth factor 2 (FGF2), lactadherin, VEGF-A, matrix metalloproteinase (MMP), and angiopoietinrelated protein 1. The WNT4 protein-rich hUC-MSC-derived exosomes have been shown to activate the Wnt/β-catenin axis to support vascular remodeling and regeneration following burn injury [42].
Skin wound healing and regeneration are supported by ERK1/2 signaling, which is activated by exosomes derived from human umbilical cord blood-derived endothelial progenitor cells (EPCs). The exosomes regulate vascular genes such as VEGF-A, COX2, and FGF2 [11]. In addition, the suppression of MMP9 expression in endothelial cells may also be responsible for the pro-angiogenic effect of exosomes derived from EPCs [43].
Numerous miRNAs, such as miR-126, miR-130a, and miR-132, have also been shown to be abundant in small extracellular vesicles in other studies. These miRNAs promote wound closure by accelerating angiogenesis [44]. Additionally, it was discovered that ADSCs exosomes contain a high concentration of miR-125a, a type of miRNA that targets delta-like canonical Notch ligand 4, an angiogenesis inhibitor, by transferring to endothelial cells [45].
Moreover, exosomal miR-21 can function on the PTEN gene to trigger the PI3K/Akt signaling pathway and the downstream mitogen-activated protein kinase (MAPK)/ERK axis, which will advance angiogenesis [46].
Cell proliferation and migration
The process of cell proliferation is necessary for the healing of wounds. Exosomes from various cells have been demonstrated in studies to hasten fibroblast and keratinocyte migration and proliferation [47]. For example, exosomes derived from bone marrow mesenchymal stromal cells and ADSCs improve the wound healing process in vitro by promoting fibroblast migration and growth in a chronic diabetic wound in a dose-dependent manner [44].
Small extracellular vesicles also promote skin cell proliferation and are associated with increased levels of proliferating cell nuclear antigen, cytokeratin 19 and collagen I [48]. In addition, UC-MSC–derived exosomes have been shown to inhibit keratinocyte apoptosis by inhibiting the pro-apoptotic proteins Bcell lymphoma 2 (BCL2) and BCL2-like protein 4 [49].
The mechanistic target of rapamycin, MAPK, and Wnt signaling pathways have all been shown to be critical for wound healing. These signaling pathways can cause target cells to overexpress a number of growth factors, such as IGF-1, IL-6, and hepatocyte growth factor [48].
MSCs-derived synovium exosomes provide miR-126-3p overexpression to heal skin defects in a diabetic rat model. MiR-126-3p regulates the MAPK/ERK and PI3K/AKT pathways to induce the cell proliferation and migration of dermal fibroblasts [50].
It is unclear, however, how the contents of the tiny extracellular vesicles affect upstream and downstream phosphorylation in associated molecular pathways.
Conclusion
In the last few decades, MSCs have been applied in numerous studies for wound healing and regeneration medicine. As a novel therapeutic approach, exosome-based therapies can reduce the formation of scars and hasten wound healing. Proteins involved in the control of biological processes linked to wound healing are abundant in USC-exosomes. Notably, USC-exosomes exhibit high expression of DMBT1, a pro-angiogenic protein. It seems that USC-exosomes could be a potential approach for wound healing by enhancing blood vessel formation through the delivery of DMBT1 protein.