 |
 |

|
| Aims and Scope |
| About the Journal |
| About the Society (KWMS) |
| Editorial Board |
| Open Access |
| Principles of Transparency and Best Practice |
| Editorial Office |
| Subscriptions |


|
| Aims and Scope |
| About the Journal |
| About the Society (KWMS) |
| Editorial Board |
| Open Access |
| Principles of Transparency and Best Practice |
| Editorial Office |
| Subscriptions |

AbstractClay is ubiquitous, and its hydrous phyllosilicate properties have promoted its use as a traditional wound healing agent in many cultures. Various types of natural clay have been utilized for skin diseases and wound treatments. Therefore, we aimed to study natural and synthetic clay minerals and summarize their applicability in medical settings. A review of prospective studies published since 2008 examining the properties of different forms of natural clay minerals and their therapeutic benefits in wound care was conducted. Studies were obtained using the databases of Google Scholar, PubMed, Web of Science, and HINARI (Health Inter-Network Access to Research Initiative) and searching related journals. The primary outcomes were assessed qualitatively and were categorized by the type of natural clay used. The included papers studied five available types of clay: Chamliyal clay, French green clay, Oregon Mineral Technologies blue clay, Kisameet clay, and various synthetic clays. The studies found how these forms of clay together demonstrate anti-inflammatory and antibacterial properties while promoting fibroblast regeneration and circulation while remaining biocompatible. Clay-based materials may be a potential alternative for conventional dressings for wound healing in resource-limited settings. This review opens doors to expanding clay-based therapies for wound healing.
IntroductionClay is a natural substance found worldwide that is composed of minerals. Carbonates, silicates, hydrated oxides of iron and aluminum, and other various organic and inorganic elements are typically linked with clay [1]. There are around seventy different varieties of clay, which are classified based on their crystal-chemical properties. Hydrous phyllosilicate particles are found in clay minerals, allowing for a unique negative electrical charge [2]. Clay species have different electrical charge values. Still, they all can absorb and remove hazardous substances or toxins. Clay minerals comprise two separate sheet structures, tetrahedral and octahedral sheet components, whose proportion and organization define different clay kinds and even confer physiochemical qualities [3]. Oxygen atoms are shared by these two sheet elements, which link them and form a 1:1 or 2:1 crystalline structure. In 1:1 type clay minerals, layer structures impart strong bonds between one octahedral sheet layer and one tetrahedral sheet layer, resulting in both less hydration and swelling. Meanwhile, 2:1 type minerals share a weaker bond between two tetrahedral sheet layers, which imparts the unoccupied interlayer space the ability to absorb water and swell, resulting in mineral structure expansion. These properties help reduce excess exudates and aid in wound healing and odor control [4,5].
The types of clay that are commonly used for medical and pharmaceutical therapies include kaolinite, halloysite, montmorillonite, beidellite, talc, sepiolite, and palygorskite [1,2,6]. In general, there are five common types of clay minerals, halloysite, kaolinite, illite, montmorillonite, and smectite, as depicted in Fig. 1. They differ in their proportions and orientations of alumina and silica, as well as the identity of ions that tend to populate in the interlayer space between alumina/silica layers [1]. These clays have been categorized as modulated mineral groups; however, these clays do not impart the essential features of the continuous planar layer structures as discussed above. They form ribbon-like structures and fibers with periodically inversed tetrahedral sheets and non-continuous octahedral sheets (Fig. 2) [7-9].
Historically, natural clay has been used for a wide range of purposes. It is recorded that early Mesopotamians used clay for treatments of wounds and to stop hemorrhaging. The wound healing process occurs in four stages. It begins with bleeding, where damaged blood vessels release vasoactive compounds to initiate vasoconstriction and allow the formation of blood clots, providing a hemostatic plug and a defense barrier from infections. Next, inflammatory mediators are released, attracting monocytes which are the source of growth factors. When the acute inflammatory response subsides, the proliferation stage begins, new cells grow, leading to the final remodeling stage [10]. An understanding of the stages of wound healing is crucial because acute wounds have the potential to become arrested in the inflammatory stage slowing down the healing process and developing into chronic wounds [11].
Wound therapy aims to provide an ideal environment to facilitate the natural process of wound healing. It is mainly achieved with wound dressings and topical agents. The “perfect” wound dressing material should fulfill multiple criteria. Some of the most important features include non-toxicity, oxygen permeability, mechanical stability, biodegradability, and the ability to prevent infection [12]. Clay minerals, besides seldom causing skin abrasions, have high adsorption and hydrophilic capacities, and a high cation/anion exchange capacity promoting a reversible exchange of chemical elements. The synthetic clay laponite forms a gel characterized by texture resembling normal skin when applied to wounds. The lamellar spaces in the structure of laponite expand to intercalate different molecules, making it an ideal carrier of biomolecules such as amino acids and drugs to be delivered to the wound site and accelerate wound healing. Utilizing laponite as such a carrier of substances to enhance the wound healing process is an extremely attractive option due to its low cost, biocompatibility, large surface area due to the layered structure, eco-friendly characteristics and high cation exchange capacity [13]. In both natural and synthetic clay minerals, the colloidal size and crystalline structure allow easy formation of materials with large surface areas, high heat capacities, exploitable rheological characteristics and excellent absorptive abilities. Further, although the physical properties of clay minerals have long been recognized, it is primarily the chemical and mineralogical attributes that have been demonstrated to modulate bacteria, providing both a bactericidal and proliferative effect. Londono et al. [14] state that soluble clay constituents of Colombian clay from the Amazon, along with its metal cations such as aluminum, are essential to its antibacterial properties. And it is the combined physical properties of minerals and effect of various leached metal ions that provide broad spectrum bactericidal activity observed. Likewise, Falkinham et al. [15], observed that clay native to Jordan’s red soils was able to kill Micrococcus luteus and Staphylococcus aureus species while promoting proliferation of antibiotic-producing microbes during inoculation. Various studies highlight the ability of clay minerals to alter the pH, oxidation state, osmotic strength and temperature of the surrounding environment catalyzing the bactericidal effects. The antimicrobial effect mechanism may be due to production of hydroxyl radicals, DNA damage, inhibition of DNA replication, reduction of protein synthesis, nucleic acid degradation, irreversible binding and inhibition of biological molecules and replacement of ions that are essential for cell membrane stability [3-6,16].
The financial burden of wound care management on the healthcare economy has continued to rise; Medicare costs for acute and chronic treatment ranged from 28 to 96 billion dollars in 2018 in the United States [17]. In a surgical patient, acute wound healing failure can lead to increased morbidity and mortality [18]. No matter what field in medicine physicians practice, they will encounter and face challenges managing chronic wounds [16]. This review article advocates for more robust research of clay minerals in wound care therapy. We look at previous studies demonstrating the therapeutic benefits of clay minerals in wound care. In addition, we speculate advanced innovations with clay products will reduce wound treatment challenges in inpatient and outpatient settings and reduce healthcare costs.
MethodsA literature review of prospective studies published in various international journals from 2008 to 2022, which examined the properties of different forms of natural and synthetic clay minerals and their therapeutic benefits in wound care, was conducted. The eligibility criteria are illustrated in Table 1, and articles were searched for using search terms enumerated in Table 2. All the search results were exported to a spreadsheet for organized review, and a quality evaluation was conducted, noting the study type. Relevant articles were evaluated if they meet the desired criteria via the abstract section. If they fulfilled the criteria, the full article was downloaded for a thorough evaluation. All studies were obtained using three databases, Google Scholar, PubMed, and Web of Science, and searching related journals. Whenever the full article was not available, the HINARI (Health Inter-Network Access to Research Initiative) database was used, which was made free to low and lowmiddle-income countries like Nepal. Bibliographic data, article type, aims and context, and data collection and analysis methods were the data items collected. In studies involving metagenomic analyses, species richness and relative abundances were also considered. Sample sizes were also considered where appropriate. Bias was assessed contextually and individually, as every article did not have similar aims or methods. However, it was expected that, where appropriate, randomization and blinding was employed. Review Manager 5.3 was used to compile qualitative and quantitative results from individual articles.
ReviewClay minerals can be broadly classified as naturally occurring clay minerals and synthetic clay minerals; the latter impart the same overall structure and physicochemical properties as natural clay minerals [16]. Natural clays have been commonly termed as cationic clays whilst synthetic clay-like materials termed as hydrotalcite-like layered double hydroxides are designated as anionic clays [19]. They commonly contain layers of octahedral sheets, in which isomorphic substitution causes a positive charge to be present in the interlayer spaces, which in turn requires spectator anions to counterbalance charges, allowing the intercalation of guest species. The interlayer also allows for oxygen permeability [20]. The main benefit of synthetic clay minerals would be the possibility of large-scale production when the naturally available clays might be scarce in the forthcoming years. The conformation and unique lattices of various clays help contribute to their antibacterial and biocompatible properties. Furthermore, clays are not abrasive, making them suitable for application as a wound dressing [21].
Natural clay in wound healingChamliyal clayThe ability of clay to fight skin illness has been attributed to its chemical composition, including antibacterial and anti-inflammatory properties. This phenomenon has been explored in several studies. Sharma et al. [21] researched natural Chamliyal clay from India, also known as Shrine clay. This clay-water paste has been used as an ointment to treat skin problems such as psoriasis in northern India and Pakistan. Sharma et al. [21] noted that certain clay microorganisms within Chamliyal clay are thought to be important for skin healing. Samples were collected from random locations in Chamliyal, DNA was extracted, and gene-targeted analysis was performed. Chamliyal clay was dominant with the common microbial species (Proteobacteria sp., Actinobacteria sp., Acidobacteria sp., Firmicutes sp., Bacteroidetes sp., and fungal species) found in most clays (Fig. 3). In addition to these phyla commonly found in clay, Chamliyal clay also contains sulfate-reducing species of bacteria, Deltaproteobacteria, and iron-reducing bacteria such as Deferribacteres. Sharma et al. [21] attributed the healing properties to the metabolism of sulfur and iron.
French green clay
Mycobacterium ulcerans causes Buruli ulcers, a chronic necrotizing skin disease common in central and western Africa. The treatment for this particular ulcer usually consists of surgical excision and antibiotics [22]. Line Brunet de Courssou observed tribes from the Ivory Coast of Africa suffering from Buruli ulcers. She imported local green clay that she had used for bug bites and cuts while growing up in France. de Courssou documented that she used clay from two different suppliers. In addition, she wrote numerous cases and noticed that one sample of clay effectively killed Mycobacterium while the other sample promoted granulation tissue growth, facilitating healing once the bacteria was eliminated [16,23].
French green clay is predominantly made up of smectite and illite minerals. Haydel et al. [24] studied broad-spectrum antibacterial properties of two minerals found in French clay. CsAgO2 and CsArO2 cesium oxides are often adsorbed onto the iron-rich clay minerals smectite, and illite [25]. CsAgO2 illite is enriched with magnesium and potassium, while CsArO2 illite clay mineral is enriched with calcium. The study concluded that CsAgO2 in a liquid medium demonstrated bactericidal activity against Escherichia coli, Salmonella enterica, Pseudomonas aeruginosa, and Mycobacterium marinum; and reduced growth of methicillin-resistant S. aureus and Mycobacterium smegmatis. The CsArO2 mineral components had no such effect, or it increased the growth of bacteria. They conclude that a combination of elements in suitable medium lead to the antibacterial activity of CsAgO2 as opposed to a single component [23]. The study proposed that the results noted with CsArO2 were related to montmorillonite clay mineral composed of smectite and kaolinite. Montmorillonite and additional clay minerals can protect environmental bacteria and stimulate bacterial growth. This study supports the previous finding of de Courssou, where CsAgO2 eliminated certain bacteria while CsArO2 promoted cell growth and granulation tissues [22,23].
OMT blue clayCaflisch et al. [26] studied Oregon Mineral Technologies (OMT) blue clay. Predominately made up of illite-smectite, quartz, pyrite, and calcium-plagioclase, the illite-smectite contains an expandable structure that behaves as a reservoir for metals; in an aqueous environment, it undergoes cation exchange that promotes an antibacterial effect. The study observed that hydration of OMT blue clay led to the dissolution of iron and aluminum. This reaction damaged bacterial membranes and caused intracellular protein damage via oxidation. The clay treatment demonstrated susceptibility to both Gram-positive and Gram-negative bacteria and biofilm-producing bacteria traditionally resistant to antibiotics [26].
Kisameet clayBehroozian et al. [27] studied in vitro antibacterial activity of Kisameet clay from Canada on multidrug-resistant bacteria strands. This clay was traditionally used for centuries to treat burns, arthritis, phlebitis, etc. Unlike most natural clays, Kisameet clay contains lower amounts of clay minerals and is predominately made up of biotite minerals. They suggested the clay’s antimicrobial activity to ESKAPE pathogens (E. faecium, S. aureus, K. pneumonia, A. baumannii, P. aeruginosa, and Enterobacter species) is related to its extensive resident microbial community [27]. In addition, Actinobacteria are found in this community and can produce bioactive molecules that amplify the antimicrobial effect (Fig. 3).
Synthetic clay in wound healingSynthetic clay is being more widely used in a variety of industries. Multiple investigations have been conducted on montmorillonite and laponite for biological applications and drug delivery in synthetic clays [20]. Montmorillonite, commonly known as bentonite, is a naturally occurring clay that has been used to treat various diseases, including poison ivy contact dermatitis [28]. The antibacterial activities of copper and zinc loaded on montmorillonite were investigated by Jiao et al. [29]. Montmorillonite was chosen for its high absorption capacity and ability to eliminate toxins from the skin and digestive tract. According to their findings, montmorillonite loaded with copper and zinc in various ratios had a synergistic antibacterial activity, while also demonstrating increased cytotoxicity with increased concentrations of copper and zinc [29]. Tomas et al. [30] reviewed the potential of laponite in drug delivery and tissue repair. Laponite is a synthetic clay that exhibits structural similarity to the natural clay hectorite. Laponite also contains nanoscale crystals that allow interactions with various chemicals, making it suitable as a carrier for transporting other molecules. Furthermore, this synthetic clay can exist as a self-organized gel form. When bone marrow stroma, vascular endothelial growth factors, or other differentiable cells were incorporated with this gel form, it helped facilitate the regenerative properties of those cells.
Properties of clay that can be utilized in wound healingNatural and synthetic clays possess several beneficial properties for wound healing therapy. We will take an in-depth look at other properties that can be used to advance the use of clay minerals in wound healing.
Anti-inflammatoryCervini-Silva et al. [31] studied the anti-inflammatory responses of sepiolite and palygorskite while comparing them to previous studies of halloysite clay. They focused on macrophages and neutrophils recruiting immune mediator cells. Their experiments demonstrated edema and neutrophil migration inhibition via limiting chemical transferring and inhibiting myeloperoxidase activity. Cervini-Silva et al. reasoned that the structure of such clays may have had a role in the early inhibition of edema. Cervini-Silva et al. also highlighted distinctive features among clays in modulating the inflammatory response. For example, their analysis suggested that fibrous clays had a more profound influence on cell viability as compared to halloysite. Further, they identified that between palygorskite and sepiolite, palygorskite had a greater immediate effect on reducing myeloperoxidase, but sepiolite demonstrated higher inhibition in the longer term. Their research underscores a complex, time-dependent nature of modulation of the inflammatory response in the presence of clay minerals. It is essential that we establish ways to stabilize the inflammatory response and allow the healing progression [32].
BiocompatibilityMultiple studies have shown that clay minerals cause no significant loss in cell viability [33]. Li et al. [34] studied the toxicity of nanosilicate platelets derived from montmorillonite clay. The effect of genotoxicity on hamster ovary cells was evaluated using a micronucleus assay and Salmonella gene mutation assay. The results demonstrated that natural clay minerals such as montmorillonite alone had little to no toxic effect on cells. In addition, halloysite clay has been shown to have high biocompatibility with low toxicity. The inherent negative charge in biological environments allows it to be highly hydrated for excellent biocompatibility [35].
Fibroblast regeneration/circulationWhile clay contains numerous minerals, silica is often the dominant component, ranging from 40%−50%; the nanocrystal properties of clay are thought to be derived from its silica component [36]. Silica on the skin can facilitate collagen synthesis and act as a catalyst via hydroxylation enzymes [37]. Valenti et al. [38] evaluated topical effects of clay on rat skin collagen synthesis. A commercially available clay mask of multiple substances, including kaolin, saccharomyces, copper, silicon, zinc, iron, and magnesium was applied and collagen production evaluated. The clay treatment was applied for seven days, and the results showed increased collagen fibers in rat skin. They concluded the optimal environment, along with ion absorption provided by the clay, may have led to increased blood flow and facilitation of collagen synthesis [38]. Studies have demonstrated hemostatic capabilities in various clay materials, such as kaolinite. QuikClot Combat Gauze (Z-Medica) promotes hemostasis via direct contact of kaolinite with blood. Upon contact, blood coagulation factor XII is activated, leading to the coagulation cascade. The kaolinite-based QuikClot gauze is used by all branches of the US military [39].
Antibacterial propertiesWound infections disturb and delay the wound healing process. Clay minerals possess metal complexes and cations capable of attracting toxins and absorbing pathogens, enhancing the wound healing process. The adsorptive properties of various clays have been exploited in delivering antimicrobial drugs to various wounds. Ghadiri et al. [13] developed a Laponite-mafenide composite for application in burn wounds and studied its wound dressing properties, antimicrobial efficacy, and cytotoxicity. They found that the composite reduced the cytotoxic effect of mafenide on fibroblast cells due to Laponite’s Mg2+ complexes. The composite can be suitable as a dressing gel for more effective mafenide delivery.
DiscussionThe studies identified four distinct natural clays of interest that have been lauded traditionally for their wound-healing properties and present promise in developing wound dressings. Both Chamliyal and Kisameet clay are rich in Actinobacteria, which contributes to their antimicrobial properties [21,26]. The illite-smectite conformations of French green clay and blue clay can explain why the two clays are rich in trace minerals and suggest that illite-smectite clays can be used as a mineral reservoir in developing wound dressings [22,25]. The antimicrobial activity of Kisameet clay against nosocomial pathogens can be utilized as a prophylactic measure against nosocomial infections in surgical wounds. As Chamliyal clay has been traditionally used to treat psoriasis in South Asian regions, this form of clay can be further studied to identify any anti-inflammatory properties [21].
The literature search revealed the features and properties of various forms of clay that make them suitable candidates for developing an ideal wound dressing. The anti-inflammatory properties of clays demonstrated in some studies can be implemented in treating inflammatory skin diseases and reducing inflammation in chronic wounds. The traditional use of clay to treat atopic dermatitis further underscores this aspect. As they are biodegradable, abundant, and nontoxic, clays are also a sustainable option for wound dressings. Given that most clays are rich in silica, wound dressings can incorporate clays with different proportions of silica to facilitate collagen synthesis and, therefore, promote wound healing [13]. In addition to adsorbing small pathogens, some clays also confer antimicrobial activity through their abundance in bacterial flora that produce antimicrobial metabolites and molecules. Moreover, studies using clay composites suggest that the structure and lattice of clays can be exploited as a medium for drug delivery [33].
The scope of this analysis is limited to previous scientific publications on various clays, of which there is scant coverage. There should be more in vitro studies involving clays from different parts of the world to identify unique features including how they interact with wound microbiota, and eventually predict their potential uses [8]. This review hinted at how the conformational properties of various clays can help them serve as an effective agent for drug delivery. Therefore, polymer studies can work to optimize wound dressing designs by exploiting the conformational diversity of clays to develop more effective clay-drug composites. Animal studies can evaluate the wound-healing capabilities of novel clay composites in vivo, which can be subsequently followed up with clinical trials [23].
ConclusionThe ideal wound dressing is nontoxic, prevents infection, and promotes oxygen permeability, mechanical stability, and biodegradability. Clay minerals carry some of these properties through their adsorptive, absorptive, and biodegradable features, in addition to facilitating cation/anion exchange and being less abrasive. For this reason, clays have been traditionally lauded and used in different parts of the world to treat wounds and stop hemorrhaging. Industrial clays are still used today in the clinical setting, yet these clays are not optimized conformationally to help reduce excess exudates and promote odor control. However, past ethnopharmacological studies have pointed to traditional clays with the potential to address the challenges. Clay-based materials derived from Chamliyal clay, French green clay, blue clay, and Kisameet clay may be a potential alternative for developing the ideal wound dressing, especially in resource-limited and remote settings. While the current evidence facilitates the robust use of such treatment options, further multicenter studies and research are sought to prove its applicability globally.
AcknowledgementsAll the authors would like to acknowledge Dr. Nitin Babel, MD, FACS for the constant support and guidance to facilitate in writing this review article.
Fig. 1.Geometric representation of common clay minerals. Halloysite, kaolinite, illite, montmorillonite, and smectite are common clay minerals. 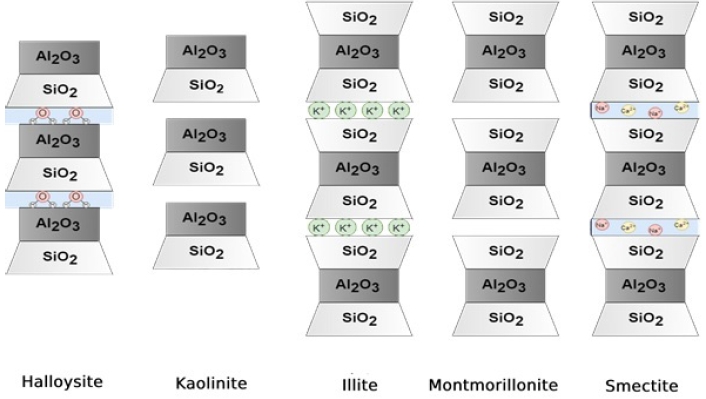
Fig. 2.Octahedral layers in 1:1 and 2:1 clay minerals. Reproduced from Christidis, 2011 with the kind permission of the European Mineralogical Union and the Mineralogical Society of the UK and Ireland [9]. 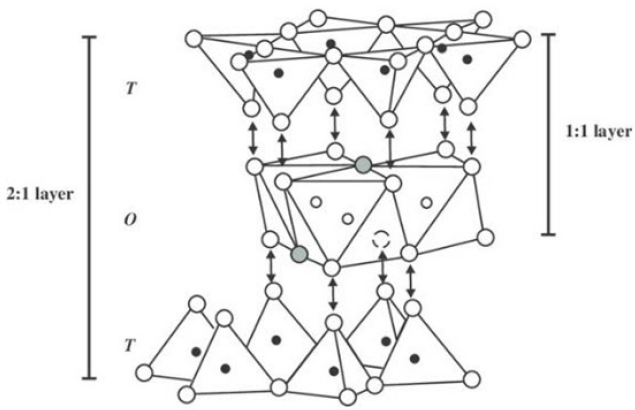
Fig. 3.Bacterial diversity of Kisameet and Chamliyal clay. Evaluated by using 16S taxonomic sequencing, Chamliyal clay (green) notably has a high abundance of Actinobacteria and sulfate-reducing bacteria compared to Kisameet clay (yellow). 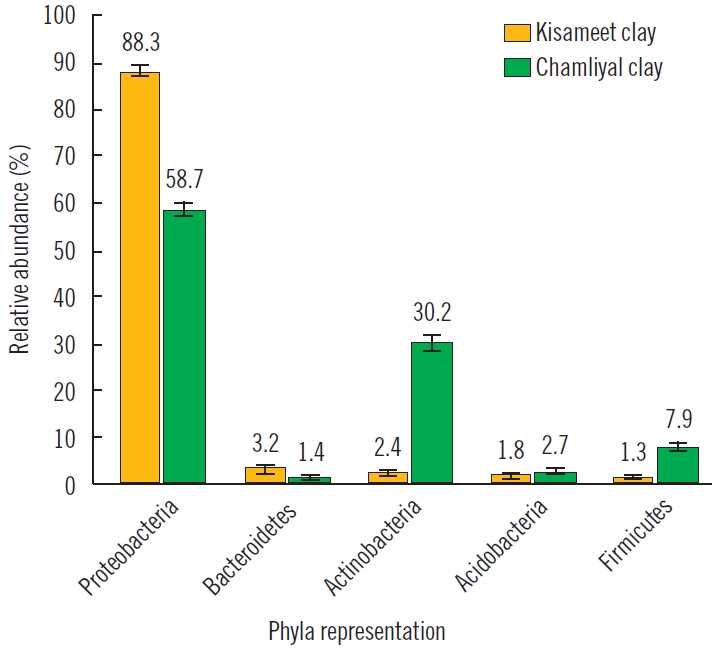
Table 1.Inclusion and exclusion criteria References1. Gomes CS. Healing and edible clays: a review of basic concepts, benefits and risks. Environ Geochem Health 2018;40:1739-65.
2. Guggenheim S, Adams JM, Bain DC, et al. Summary of recommendations of nomenclature committees relevant to clay mineralogy: report of the association internationale pour l’Etude des argiles (AIPEA) nomenclature committee for 2006. Clays Clay Miner 2006;54:761-72.
3. Brigatti MF, Galan E, Theng BKG. Structure and mineralogy of clay minerals. In: Bergaya F, Lagaly G, editors. Developments in Clay Science. Elsevier; 2013. p. 21-81.
4. Carretero MI. Clay minerals and their beneficial effects upon human health: a review. Appl Clay Sci 2002;21:155-63.
5. Choy JH, Choi SJ, Oh JM, et al. Clay minerals and layered double hydroxides for novel biological applications. Appl Clay Sci 2007;36:122-32.
6. Guo Y, Yu X. Characterizing the surface charge of clay minerals with atomic force microscope (AFM). AIMS Mater Sci 2017;4:582-93.
7. Lopez-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci 2007;36:51-63.
8. Viseras C, Aguzzi C, Cerezo P, et al. Uses of clay minerals in semisolid health care and therapeutic products. Appl Clay Sci 2007;36:37-50.
9. Christidis GE. Industrial clays. In: Christidis GE, editor. Advances in the characterization of industrial minerals. Mineralogical Society of Great Britain and Ireland; 2011. p. 341-414.
10. Hosgood G. Stages of wound healing and their clinical relevance. Vet Clin North Am Small Anim Pract 2006;36:667-85.
11. Urao N, Koh TJ. Manipulating inflammation to improve healing. In: Agren MS, editor. Wound healing biomaterials: therapies and regeneration. Woodhead Publishing; 2016. p. 117-50.
13. Ghadiri M, Chrzanowski W, Rohanizadeh R. Antibiotic eluting clay mineral (Laponite®) for wound healing application: an in vitro study. J Mater Sci Mater Med 2014;25:2513-26.
14. Londono SC, Hartnett HE, Williams LB. Antibacterial activity of aluminum in clay from the colombian amazon. Environ Sci Technol 2017;51:2401-8.
15. Falkinham JO, Wall TE, Tanner JR, et al. Proliferation of antibiotic-producing bacteria and concomitant antibiotic production as the basis for the antibiotic activity of Jordan’s red soils. Appl Environ Microbiol 2009;75:2735-41.
16. Williams LB, Haydel SE, Giese RF, et al. Chemical and mineralogical characteristics of french green clays used for healing. Clays Clay Miner 2008;56:437-52.
17. Sen CK. Human wounds and its burden: an updated compendium of estimates. Adv Wound Care (New Rochelle) 2019;8:39-48.
18. Dubay DA, Franz MG. Acute wound healing: the biology of acute wound failure. Surg Clin North Am 2003;83:463-81.
19. Gaskell EE, Hamilton AR. Antimicrobial clay-based materials for wound care. Future Med Chem 2014;6:641-55.
20. Ghadiri M, Chrzanowski W, Lee WH, et al. Layered silicate clay functionalized with amino acids: wound healing application. RSC Adv 2014;4:35332-43.
21. Sharma S, Grewal S, Vakhlu J. Phylogenetic diversity and metabolic potential of microbiome of natural healing clay from chamliyal (J&K). Arch Microbiol 2018;200:1333-43.
22. Converse PJ, Nuermberger EL, Almeida DV, et al. Treating Mycobacterium ulcerans disease (Buruli ulcer): from surgery to antibiotics, is the pill mightier than the knife? Future Microbiol 2011;6:1185-98.
23. Williams L, Holland M, Eberl D, et al. Killer clays! Natural antibacterial clay minerals. Mineral Soc Bull 2004;139:3-8.
24. Haydel SE, Remenih CM, Williams LB. Broad-spectrum in vitro antibacterial activities of clay minerals against antibiotic-susceptible and antibiotic-resistant bacterial pathogens. J Antimicrob Chemother 2008;61:353-61.
25. Kwon S, Lim J, Seoung D, et al. Comparative study of the cesium adsorption behavior of montmorillonite and illite based on their mineralogical properties and interlayer cations. J Hazard Mater Adv 2023;10:100258.
26. Caflisch KM, Schmidt-Malan SM, Mandrekar JN, et al. Antibacterial activity of reduced iron clay against pathogenic bacteria associated with wound infections. Int J Antimicrob Agents 2018;52:692-6.
27. Behroozian S, Svensson SL, Davies J. Kisameet clay exhibits potent antibacterial activity against the ESKAPE pathogens. mBio 2016;7:e01842.
28. Moosavi M. Bentonite clay as a natural remedy: a brief review. Iran J Public Health 2017;46:1176-83.
29. Jiao L, Lin F, Cao S, et al. Preparation, characterization, antimicrobial and cytotoxicity studies of copper/zinc-loaded montmorillonite. J Anim Sci Biotechnol 2017;8:27.
30. Tomas H, Alves CS, Rodrigues J. Laponite®: a key nanoplatform for biomedical applications? Nanomedicine 2018;14:2407-20.
31. Cervini-Silva J, Nieto-Camacho A, Ramirez-Apan MT, et al. Anti-inflammatory, anti-bacterial, and cytotoxic activity of fibrous clays. Colloids Surf B Biointerfaces 2015;129:1-6.
32. Landen NX, Li D, Stahle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci 2016;73:3861-85.
33. Sandri G, Bonferoni MC, Rossi S, et al. Clay minerals for tissue regeneration, repair, and engineering. In: Agren MS, editor. Wound healing biomaterials: functional biomaterials. Woodhead Publishing; 2016. p. 385-402.
34. Li PR, Wei JC, Chiu YF, et al. Evaluation on cytotoxicity and genotoxicity of the exfoliated silicate nanoclay. ACS Appl Mater Interfaces 2010;2:1608-13.
35. Vergaro V, Abdullayev E, Lvov YM, et al. Cytocompatibility and uptake of halloysite clay nanotubes. Biomacromolecules 2010;11:820-6.
36. Rahman MR, Chang Hui JL, Hamdan SB. Introduction and reinforcing potential of silica and various clay dispersed nanocomposites. In: Rahman MR, editor. Silica and clay dispersed polymer nanocomposites. Woodhead Publishing; 2018. p. 1-24.
37. Araujo LA, Addor F, Campos PM. Use of silicon for skin and hair care: an approach of chemical forms available and efficacy. An Bras Dermatol 2016;91:331-5.
|
|