Introduction
Local anesthetics mixed with epinephrine, such as lidocaine–epinephrine or articaine–epinephrine, are used to dull pain as well as to achieve hemostasis during surgical procedures. Their potential to cause necrotic changes due to irreversible vasospasm in the injected area remains controversial. It has been reported that lidocaine–epinephrine can be safely used without causing complications such as ischemia [1,2].
Articaine (4-methyl-3-[2-(propylamino)-propionamido]-2-thiophene-carboxylic acid methyl ester hydrochloride) is a local anesthetic with a thiophene ring that is commonly combined with epinephrine as it provides greater lipid solubility as compared with lidocaine. The thiophene ring facilitates diffusion across the lipid-rich nerve membrane to access target receptors [3]. However, articaine reportedly can cause skin necrosis in various regions such as the palate, eyelids, chin, or fingers [4-7].
Relatively few studies have been published regarding local anesthesia-induced skin necrosis over the trunk. Herein, we report a case of dermal necrosis observed on the iliac spine after injecting articaine–epinephrine during iliac bone grafting. The report was approved by the Institutional Review Board of Gwangmyeong Sungae Hospital (IRB No. 2023-N-006). The patient provided written informed consent for the publication of this case report.
Case
A 32-year-old woman without any relevant previous medical history presented to our clinic with non-union of the left index finger. An iliac bone graft was planned to manage the bone gap. Surgery was performed under general anesthesia. To promote hemostasis at the donor site, 8.5 mL of articaine hydrochloride (68 mg/ampoule) and epinephrine (30.6 μg/ampoule) (Huons Articaine Epinephrine Inj) were injected subcutaneously. A 3-cm skin incision was made over the left iliac crest area and dissection carefully proceeded through Camper’s and Scarpa’s fascia until the iliac crest was reached. The patient was 157 cm tall and weighed 51 kg for a body mass index (BMI) of 20.69 kg/m², and had a minimal amount of subcutaneous fat tissue. Therefore the iliac crest periosteum was easily approached without excessive undermining and a window was made in the cortex with an electric saw, after which 2 cc of cancellous bone was harvested, and gel was placed into the harvested area for hemostasis. After hemostasis was achieved, we used continuous sutures to close the periosteum and subcutaneous tissue with PDS 4-0 and the skin with nylon 5-0. During the surgical procedure, any bleeding observed while incising or dissecting was controlled by gently applying gauze. No abnormal persistent bleeding was identified in any specific area. After suturing the skin, we assessed the skin’s blood circulation to identify any potential issues caused by excessive suture tension. The color and capillary refill time of the sutured skin appeared normal, with no visible signs of impaired blood circulation.
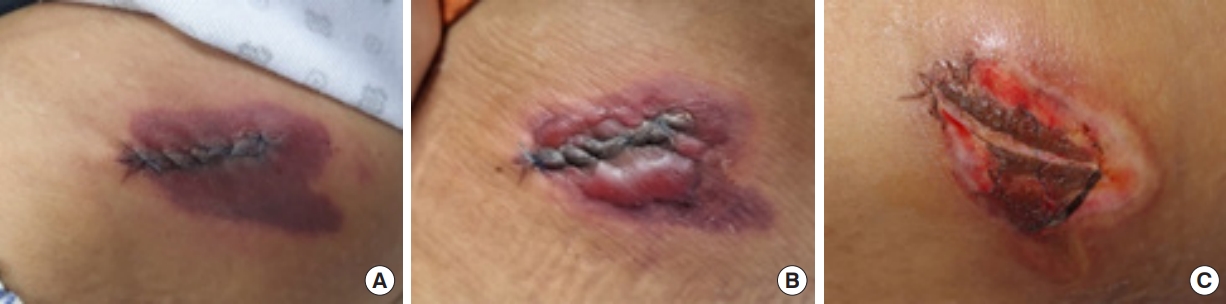
The patient was placed on bed rest and was encouraged to use compression bands at the donor site to prevent the development of hematomas and reduce pain. On the first postoperative day, we observed epidermal level demarcation around the iliac crest suture site (Fig. 1A). The patient reported pain and tenderness; however, there were no evident signs of hematoma formation or inflammation such as erythema or warmth.
On the second postoperative day, a bulla was observed around the suture margin (Fig. 1B) and was ruptured through a needle incision. There was no recurrence; however, the initial bulla formation led to a loss of skin elasticity, subsequently resulting in the spontaneous release of stitches. Necrosis was identified around the suture margin dermal level. No other signs of inflammation were observed during the 7-day postoperative period, and the area of color change observed on the first postoperative day had not extended.
The only significant laboratory finding was a mild elevation of the C-reactive protein level to 1.99 mg/dL. Hence, the patient was conservatively managed with intravenous antibiotics and anti-inflammatory medications. At follow-up 2 weeks after surgery, there had been no significant extension of the marginal necrotic changes (Fig. 1C).
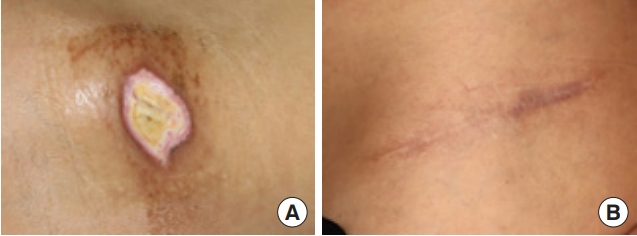
Two months later, after injecting 2% lidocaine without epinephrine, debridement of the dermal necrotic tissue over the iliac crest was performed with secondary closure of the dehisced surgical wound (Fig. 2A). In the 6-month post-procedure follow-up, the surgical site had healed without any necrotic changes (Fig. 2B).
Discussion
The iliac crest is commonly regarded as one of the most suitable donor sites for bone grafts and is nourished by the ascending branch of the deep circumflex iliac artery, which typically originates from the external iliac artery [8,9]. The average diameter of the ascending branch of the deep circumflex iliac artery measures over 4.3 mm and courses between the internal oblique and transverse abdominal muscles to reach the periosteum of the iliac crest [10]. While the precise configuration of vessels in the subdermal plexus cannot be ascertained without appropriate evaluation such as angiography or Doppler sonography, the iliac crest area is generally recognized to have a rich blood supply. The occurrence of superficial or deep hematomas at the donor site is a common complication following iliac bone grafting [11]. To mitigate intraoperative bleeding, postoperative pain, and the risk of hematoma formation, local anesthetics mixed with epinephrine, such as articaine–epinephrine (articaine hydrochloride combined with epinephrine), are commonly administered at the surgical site.
There have been several reports of skin necrosis resulting from epinephrine-mixed local anesthetic injections, including cases of delayed-onset digital ischemia after lidocaine–epinephrine injection, eyelid necrosis induced by local anesthesia injection, and scrotal skin necrosis after lidocaine–epinephrine injection [4-6]. Torrente-Castells et al. [7] were the first to report a case of skin necrosis after articaine–epinephrine injection, which was observed on the right side of the lower lip and chin following an inferior intra-alveolar nerve block. A case of hard palate mucosal necrosis following injection has also been documented [12].
Most vessels in the superficial and deep dermal plexus consist of high-resistance terminal arterioles; papillary loops (true capillaries) and post-capillary venules are usually situated in the superficial papillary dermis 1–2 mm beneath the epidermal surface [13]. Terminal arterioles are innervated by efferent sympathetic neurons, allowing for precise control of skin blood flow.
The development of skin necrosis subsequent to articaine–epinephrine injection can be explained through various mechanisms. The direct injection of articaine–epinephrine into an artery may trigger muscle contractions by activating alpha-adrenergic receptors [14]. If this inadvertently leads to persistent ischemia, tissue necrosis can ensue. Sympathetic nerve stimulation by a local anesthetic may also induce vasospasms, resulting in focal necrosis. Irritation of the arterioles by needles could also cause ischemic damage due to vasospasms.
Traction during the surgical procedure might be another potential cause of postoperative skin necrosis. However, the 3-cm incision was sufficient for identifying the iliac crest region without excessive traction. Furthermore, as we obtained only 2 cc of cancellous bone and no cortical bone at all, the harvesting procedure took less than 10 minutes. Additionally, since the patient was a 32-year-old female with a normal BMI of 20.69 kg/m², we could easily access the iliac crest with minimal undermining. Therefore, we considered traction injury or excessive undermining to be less likely causes of skin necrosis.
Several other factors could contribute to dermal necrosis. The infiltration of excessive volumes of local anesthetics has been proposed as another factor leading to necrosis, as it elevates extravascular pressure and leads to vessel occlusion. In this particular case, we injected 8.5 mL of articaine hydrochloride (68 mg/ampoule) and epinephrine (30.6 μg/ampoule) evenly and slowly, infiltrating around the 3-cm incision site with a 1-cm margin. The total injected area measured approximately 3×2 cm. It should be noted that the volume of the injected solution cannot be considered excessive since it was administered in the trunk region rather than the extremities, allowing the solution to disperse immediately without accumulating in a single area.
Skin tension along the suture site could also result in poor circulation along the wound. However, we did not observe any excessive tension on the stitches during our postoperative monitoring (Fig. 1A).
Previous studies have suggested infection as another potential risk factor for the development of skin necrosis [15]. However, in the present case, no significant signs of inflammation were observed except for mild swelling. There was no apparent discharge or erythematous change at the surgical site. To prevent wound infection and facilitate healing, we applied mupirocin ointment (Esroban Oint; JW Shinyak) with daily foam dressings (MediTouch; Ildong Pharmaceutical Co., Ltd.). We also recommended gentle compression on the donor site with an abdominal band to reduce pain and prevent swelling.
Pre-existing microcirculation damage has also been reported as a possible cause of necrosis. The patient had no significant medical history, and her blood pressure remained stable, with the systolic blood pressure ranging from 100 to 120 mmHg throughout the entire procedure. Therefore, we considered the patient’s overall preoperative and intraoperative condition to be unrelated to skin necrosis.
Our study has some limitations. Firstly, it is worth noting that we did not continuously monitor the skin circulation on the day of the surgery. Considering the patient’s baseline condition and the surgical context mentioned above, there were no discernible factors that might lead to compromised blood circulation. Furthermore, there were no prior reports of skin necrosis resulting from the administration of anesthesia in the iliac crest area. Consequently, we did not monitor skin circulation immediately after surgery. Had we assessed the skin condition on that same day, taking into account the known action time of articaine, it could potentially have revealed compromised circulation [3]. The unavailability of such observations remains a limitation. Moreover, as we did not administer any reversal medications, such as phentolamine, known to prevent necrotic changes after local anesthesia-induced ischemia in fingers, we cannot present treatment options for local anesthesia-induced skin necrosis. With the known half-life of articaine solution being 20 minutes, we believe that if we had observed continued deterioration of blood circulation after surgery and administered phentolamine accordingly, it could have provided a more direct means to confirm the causality of articaine [4]. Additionally, as our facility discontinued the use of articaine–epinephrine following this event, we are unable to provide statistical analysis to establish a relationship between articaine–epinephrine and skin necrosis.
This report aims to highlight the occurrence of skin necrosis cases after the injection of a commonly administered epinephrine-mixed articaine anesthetic in the iliac crest. Our case underscores the importance of monitoring the circulatory status following anesthesia administration in the trunk area, in order to enhance anesthetic efficacy and surgical convenience, and ultimately ensure patient safety.