 |
 |

|
| Aims and Scope |
| About the Journal |
| About the Society (KWMS) |
| Editorial Board |
| Open Access |
| Principles of Transparency and Best Practice |
| Editorial Office |
| Subscriptions |


|
| Aims and Scope |
| About the Journal |
| About the Society (KWMS) |
| Editorial Board |
| Open Access |
| Principles of Transparency and Best Practice |
| Editorial Office |
| Subscriptions |

AbstractOpen wounds with denuded bone may pose a challenge for surgeons if primary closure or flap surgery is not feasible. In such cases, cortical bone fenestration (CBF) has been employed as a preconditioning procedure to induce granulation tissue growth and provide a suitable bed for skin grafting or secondary intention healing. Our clinical study aimed to assess the outcomes of CBF performed on open wounds involving the denuded frontal bone. We treated a patient with multiple open wounds involving the denuded frontal bone using secondary intention healing in conjunction with CBF procedures and evaluated the outcomes of the fenestrated bones as well as the wound healing process at regular intervals. Granulation tissue did not develop on the fenestrated bones throughout the healing process. However, the frontal wounds healed well without complications at 16 weeks after CBF through the centripetal migration of granulation tissue from the wound margins and simultaneous epithelialization. Our results suggest that CBF procedures that do not adequately expose the diploic space fail to induce granulation tissue growth and may not be needed to treat open wounds with less than 5 cm of denuded skull bone.
IntroductionSkull bone devoid of periosteum is often exposed following tumor excision, trauma, or burns. Proper management is necessary because open wounds with exposed denuded bone are at risk for delayed healing and complications. Such wounds have been managed using various treatment modalities, with options including surgical repair and secondary intention healing. However, open wounds with denuded bone may pose challenges for surgeons if primary closure or flap surgery is infeasible because the wound is unsuitable for skin grafting. In such cases, cortical bone fenestration (CBF) has been performed as a preconditioning procedure to induce granulation tissue growth and provide a suitable bed for skin grafting or secondary intention healing. Some surgeons [1-5], who perform Mohs surgery, have favored secondary intention healing along with CBF, even when surgical repair is possible. On the other hand, others [5-13] have preferred skin grafting following CBF, though some of them have applied artificial dermis [9-11] or acellular dermal matrix [13] to the fenestrated bone before performing skin grafting in order to ensure durable coverage. Most surgeons have emphasized that CBF is a crucial procedure for managing open wounds with denuded skull bones and have reported that it stimulates granulation tissue, regardless of its depth.
In this study, we treated a patient with multiple open wounds involving denuded frontal bone using secondary intention healing after CBF procedures. We evaluated the outcomes of the fenestrated bones and the healing process of the open wounds to determine whether CBF on the denuded frontal bone induced granulation tissue growth and its role in treating such wounds. The study was approved by the Institutional Review Board of Kanwon National University Hospital (No. KNUH-2020-09-008-001) and the patient provided written informed consent for the publication of this case study.
CaseA 77-year-old man was admitted to the department of neurosurgery at our hospital to undergo surgery for an intracranial meningioma. Upon admission, a 2.5×3 cm ulcerative lesion was incidentally found on the left parietal scalp and confirmed as squamous cell carcinoma by histopathologic examination. A joint operation was planned between neurosurgery and plastic surgery to excise the skin cancer on the scalp and remove the intracranial tumor. The incision line was designed preoperatively considering the next-step repair of the scalp defect after excision of the skin cancer, as well as flap elevation for the removal of the intracranial tumor (Fig. 1).
With the patient under general anesthesia, his skull was fixed in a prone position. First, the skin cancer on the scalp was excised, including the underlying periosteum, in accordance with the preoperative design. A rectangular scalp flap measuring 8×11 cm was elevated over the periosteum anterior to the scalp defect and transposed onto the scalp defect. The donor site of the scalp flap on the frontal area was grafted using 1:3-meshed split-thickness skin taken from the left lateral thigh. The graft was immobilized with a tie-over dressing. Next, the neurosurgeon successfully removed the intracranial tumor through elevation of the preoperatively designed U-shaped flap and closed the surgical wound primarily.
Two weeks after surgery, the grafted skin showed multifocal loss with denuded frontal bone exposure (Fig. 2). The largest area of denuded bone had dimensions of about 3.5×4.5 cm. The areas of exposed bone were fenestrated using a high-speed surgical drill with a rose-head burr. Multiple burr holes were made with variable depths until minor bleeding occurred. After the bone fenestration surgery, the wound was kept clean and moist by employing a regular simple dressing with an antibiotic ointment.
A computed tomography (CT) scan obtained 2 weeks after the procedure showed the variable depths of fenestration (Fig. 3). No granulation tissue was observed on the areas of fenestrated bone throughout the entire follow-up period (Fig. 4). Over time, the fenestrated regions became shallow as new bone formation proceeded (Fig. 5). The frontal wounds healed well at 16 weeks after CBF through the centripetal migration of granulation tissue from the wound margins and simultaneous epithelialization without complications. A follow-up examination at 18 months demonstrated durable coverage on the frontal bone.
DiscussionCBF has commonly been used as a preconditioning procedure to treat open wounds with exposed skull bones. Surgeons have utilized a variety of techniques and depths to fenestrate the outer table of the calvarium. Many surgeons [3,5,8,10-13] have employed a surgical drill to create multiple burr holes, while others have opted for methods like chisels [2,3] or lasers [9] to partially or fully remove the outer cortex. Most surgeons fenestrated the outer cortex until minor bleeding occurred, subsequently reporting the observation of granulation tissue growth within a few weeks. Latenser et al. [5] also noted that complete fenestration of all exposed bone is not always necessary to stimulate granulation tissue. This is because the outer table of the skull contains a robust Volkmann’s and Haversian system, although tissue growth is influenced by factors including the depth and breadth of fenestration, the degree of mesenchymal tissue stimulation, and the richness of the blood supply tapped into. In contrast, Becker et al. [4] reported that they did not routinely perform CBF because granulation tissue typically develops from the Haversian canals and wound edges in most patients.
In our clinical case, multiple CBF procedures did not induce granulation tissue growth. Nevertheless, the open wounds with frontal bone exposure healed well through the centripetal migration of granulation tissue from the wound margins and simultaneous epithelialization. Similarly, Becker et al. [4] successfully treated 38 Mohs surgery wounds with exposed bone on the scalp and forehead using secondary intention healing without CBF. Therefore, it appears that CBF might not be an essential procedure for small lesions (less than 5 cm), even though healing might take longer than in wounds that heal with the assistance of granulation tissue formed on fenestrated bone. However, CBF procedures may be crucial for larger lesions. It is uncertain why our CBF procedures did not induce granulation tissue formation, but one reason might be that the depth of fenestration was too shallow to induce granulation tissue, even though CT showed a variable depth of fenestration: some burr holes fenestrated into the diploic space, while others did not.
The outer table of the skull is nourished by the blood supply of the Haversian system as well as the periosteum. Barry et al. [3] reported that small bleeding points indicate the correct depth has been attained in terms of reaching the diploic space. However, this claim seems not to hold true because minor bleeding can occur only when exposing the Haversian system, not the diploic space. The diploic space refers to the marrowcontaining area. Mesenchymal stem cells derived from bone marrow can differentiate into various cell lineages, including fibroblasts, osteoblasts, chondrocytes, and adipocytes [14]. Furthermore, recent studies suggested that bone marrow-derived stem cells such as mesenchymal stem cells, progenitor cells such as endothelial progenitor cells, and fibrocytes may be involved in optimal cutaneous wound healing; these stem and progenitor cells may be mobilized to leave the bone marrow, migrate to injured tissues, and participate in repair and regeneration [15].
While our study did not investigate the optimal conditions for CBF to stimulate granulation tissue growth, we believe that exposing an adequate diploic space through fenestration of the full-thickness outer table is necessary to induce granulation tissue growth. Failing to do so might lead to the stimulation of new bone formation instead of granulation tissue, as demonstrated in our case. The adequate size and interval of fenestration necessary to induce granulation tissue growth have not been established in previous studies. However, based on our experience, we speculate that diploic space exposure larger than 5 mm is required to stimulate granulation tissue. The interval between fenestrations might vary depending on the fenestration size, although a distance of 5–10 mm was recommended [3].
Our patient experienced no complications related to CBF. Although rare, complications such as bone desiccation, bleeding, dura perforation, osteomyelitis, cerebrospinal fluid leakage, and intracranial infection can occur [3]. Moreover, it is important to exercise caution when performing CBF to prevent unexpected events due to morphological changes in both compact and spongy bones that occur with increasing age. Preoperative CT scans should be conducted routinely, as the calvarium might sometimes be thinner than anticipated or lack the inner table and/or diploe [9]. Potential complications can also be mitigated through meticulous wound care and by avoiding the procedure in patients at an elevated risk of infection.
Based on our clinical results, CBF procedures that do not provide adequate exposure to the diploic space may fail to induce granulation tissue formation, and CBF may not be an essential procedure for treating open wounds with less than 5 cm of denuded skull bone. Further research is needed to establish the optimal conditions for CBF to stimulate granulation tissue growth.
Conflict of InterestKunyong Sung is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported. Fig. 1.Preoperative photograph. Red line: the design for skin cancer excision. Black circle: the location of the intracranial tumor. Black line: flap elevation design for removing the intracranial tumor. Blue line: the design of the transposition flap to repair the scalp defect caused by excision of the skin cancer. 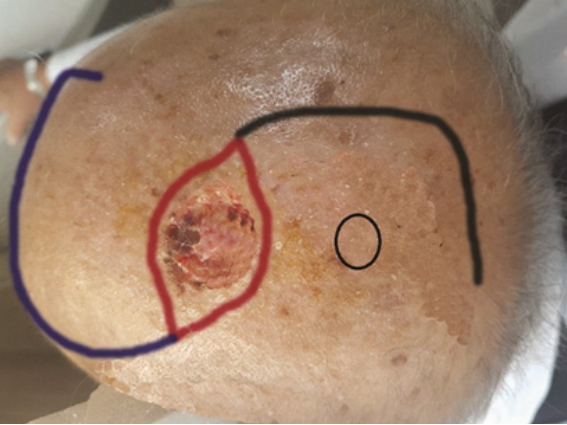
Fig. 2.Two-week postoperative photograph. Multiple areas of denuded frontal bone (black asterisks) were exposed due to the loss of grafted skin at the donor site of the transplantation flap. 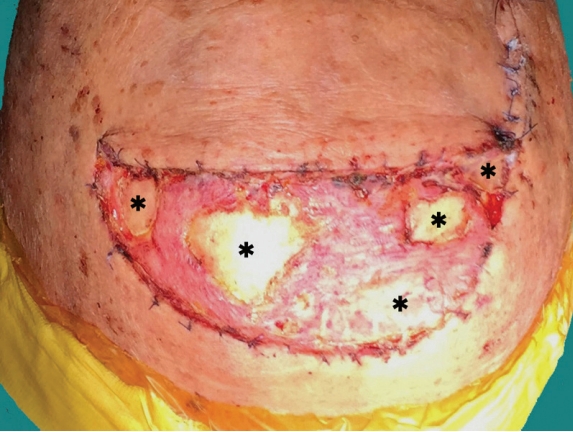
Fig. 3.Computed tomography images obtained 2 weeks after surgery. After multiple cortical bone fenestration procedures. (A) Coronal view. (B) Axial view. 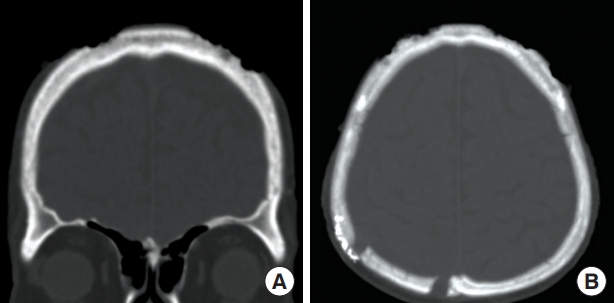
References1. Vanderveen EE, Stoner JG, Swanson NA. Chiseling of exposed bone to stimulate granulation tissue after mohs surgery. J Dermatol Surg Oncol 1983;9:925-8.
2. Snow SN, Stiff MA, Bullen R, et al. Second-intention healing of exposed facial-scalp bone after mohs surgery for skin cancer: review of ninety-one cases. J Am Acad Dermatol 1994;31(3 Pt 1):450-4.
3. Barry RB, Langtry JA, Lawrence CM. The role of cortical bone fenestration in the management of mohs surgical scalp wounds devoid of periosteum. Br J Dermatol 2009;160:1110-2.
4. Becker GD, Adams LA, Levin BC. Secondary intention healing of exposed scalp and forehead bone after mohs surgery. Otolaryngol Head Neck Surg 1999;121:751-4.
5. Latenser J, Snow SN, Mohs FE, et al. Power drills to fenestrate exposed bone to stimulate wound healing. J Dermatol Surg Oncol 1991;17:265-70.
7. Furlanetti LL, de Oliveira RS, Santos MV, et al. Multiple cranial burr holes as an alternative treatment for total scalp avulsion. Childs Nerv Syst 2010;26:745-9.
8. Terzioglu A, Aslan G, Saydam M. Trephination in the treatment of scalp avulsion: successful application of a historical method. J Oral Maxillofac Surg 1999;57:204-6.
9. Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the Erbium:YAG laser as an alternative to the rose head burr. Dermatology 2015;230:276-81.
10. Corradino B, Di Lorenzo S, Leto Barone AA, et al. Reconstruction of full thickness scalp defects after tumour excision in elderly patients: our experience with Integra dermal regeneration template. J Plast Reconstr Aesthet Surg 2010;63:e245-7.
11. Koenen W, Goerdt S, Faulhaber J. Removal of the outer table of the skull for reconstruction of full-thickness scalp defects with a dermal regeneration template. Dermatol Surg 2008;34:357-63.
12. Pitkanen JM, Al-Qattan MM, Russel NA. Immediate coverage of exposed, denuded cranial bone with split-thickness skin grafts. Ann Plast Surg 2000;45:118-21.
13. Jung SN, Chung JW, Yim YM, et al. One-stage skin grafting of the exposed skull with acellular human dermis (AlloDerm). J Craniofac Surg 2008;19:1660-2.
|
|