Introduction
Pressure ulcers are relatively common but challenging complications that are often experienced by older patients with conditions such as paralysis. Their prevalence rates vary by country; according to a study conducted in the United States, pressure ulcers were reported in 15% of hospitalized patients, 25% of intensive care unit patients, 30% of patients aged 71 years or older, and 25% to 85% of patients with neurological disorders [1]. Pressure ulcers occur when prolonged pressure and friction are applied to protruding parts of the body, leading to localized blood flow reduction, tissue damage, and necrosis.
In cases where pressure ulcers are deep or are neglected for extended periods, they can lead to abscess formation due to repeated infections and severe inflammation. Pressure ulcers with abscesses require surgical treatment for removal with their associated necrotic tissues [2]. Prior to surgery, physical examinations and imaging tests are necessary to establish a surgical plan. However, in cases without specific symptoms, as it can be challenging to suspect an abscess based solely on physical examination, they might be overlooked. Although cases of abscess formation due to pressure ulcers are well known, there have been no reported cases of an abscess extending to the thigh due to a pressure ulcer in the sacral area. In this study, we present a case in which a patient presented with a severe pressure ulcer in the sacral area and an abscess extending to the thigh. We suspected an abscess in an area without pressure ulcers and conducted an evaluation before surgery that involved debridement and reconstruction, with excellent results. We report this case to highlight the importance of considering abscesses in areas without pressure ulcers and conducting appropriate preoperative evaluations. The report was approved by the Institutional Review Board of Hallym University Sacred Hospital (IRB No. 2023-07-006) and informed consent for publication of the study was obtained from the patient.
Case
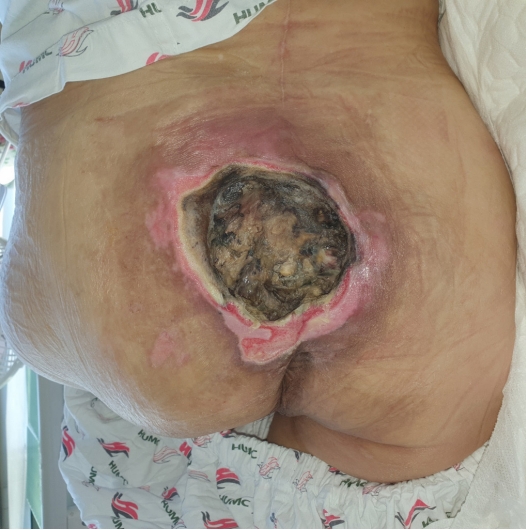
A 53-year-old man presented to our hospital with a large sacral pressure ulcer. The patient had sustained a spinal cord injury resulting in paraplegia due to a fall at a construction site 5 months prior and had a history of underlying diabetes. The pressure ulcer developed several days after the spinal cord injury and continued to deteriorate due to prolonged immobility. The size of the pressure ulcer was approximately 10×10 cm, and it was covered with necrotic tissue that emitted a strong odor (Fig. 1). The area surrounding the ulcer showed erythema and had an elevated temperature. Upon admission, blood tests revealed a C-reactive protein (CRP) level exceeding 300 mg/L, a white blood cell count (WBC) of 24,700/μL, and the patient had a body temperature of over 38.5 °C, indicating severe inflammation that required the immediate administration of antibiotics as well as surgical intervention. Empirical treatment with piperacillin 4 g/tazobactam 0.5 g every 8 hours was immediately initiated, and a wound culture was conducted. The culture results revealed the presence of Enterococcus faecium and Escherichia coli. Following the antibiotic susceptibility test results, the regimen was adjusted to tigecycline 50 mg every 12 hours.
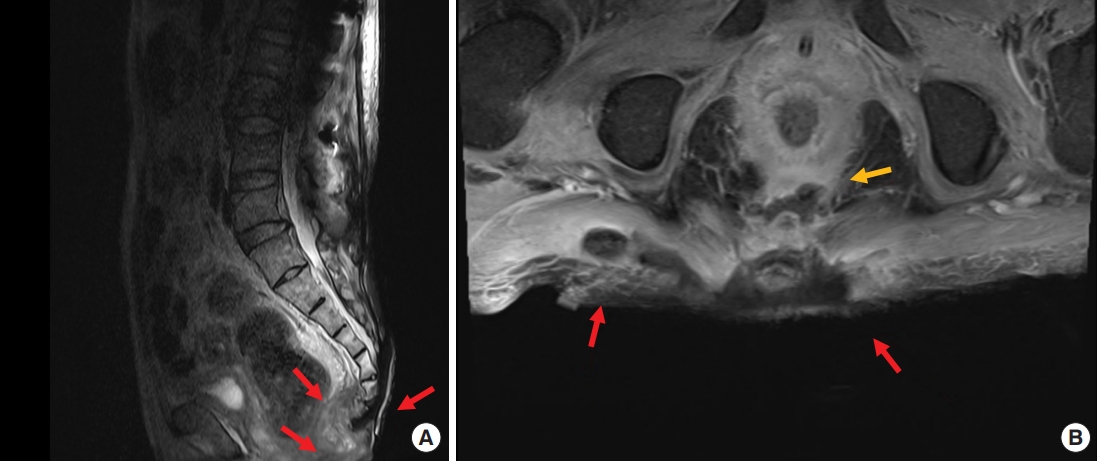
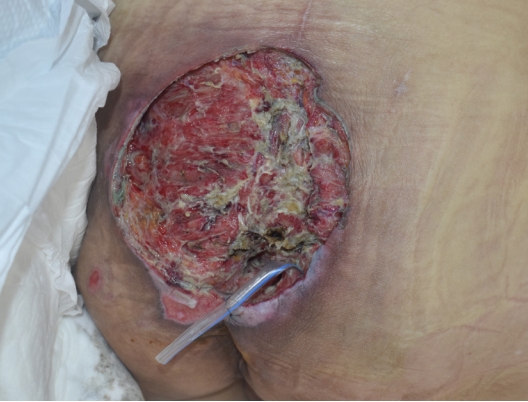
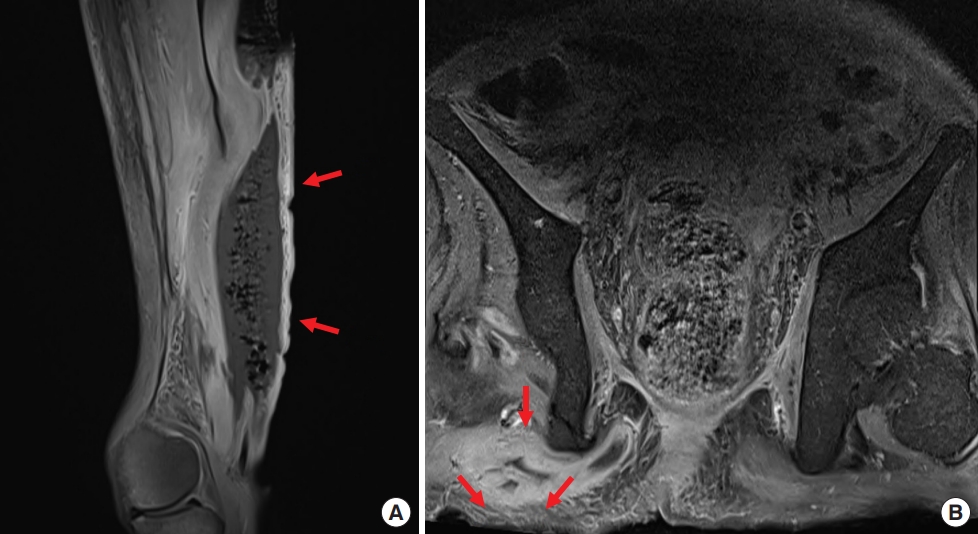
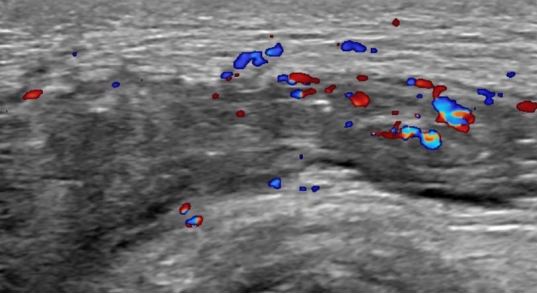
Contrast enhanced magnetic resonance imaging (MRI) was performed to investigate suspected skin and soft tissue abscesses, revealing a substantial 15×16-cm abscess spanning from the precoccygeal area to the posterior sacral area with prominent osteomyelitis of the coccyx (Fig. 2). Debridement of the pressure ulcer was performed along with coccygeal osteomyelitis ostectomy, removing the entire abscess including the precoccygeal part and most of the necrotic tissue; however, continuous pus drainage was observed in the right ischial area (Fig. 3). To rule out the possibility of an additional abscess, further physical examination was conducted on the right thigh. There were no noticeable skin lesions such as erythema, blisters, or ulcers, and the area felt smooth, but the back of the right thigh was slightly swollen. Though the physical examination did not likely indicate a thigh abscess, additional MRIs of the right thigh and the sacral area were performed to rule out such possibility. MRI scans showed a 9×21-cm abscess invading the right hamstring muscle of the posterior thigh, with communication between the thigh abscess and the pressure ulcer through an ischial region fistula tract (Fig. 4). In response, an aspiration culture was conducted from the right thigh abscess, revealing the presence of the same bacteria as isolated from the pressure ulcer. Ultrasonography of the right ischial area also confirmed the presence of a fistula tract located in the piriformis muscle, connected to the sacral area and the posterior aspect of the right thigh (Fig. 5).
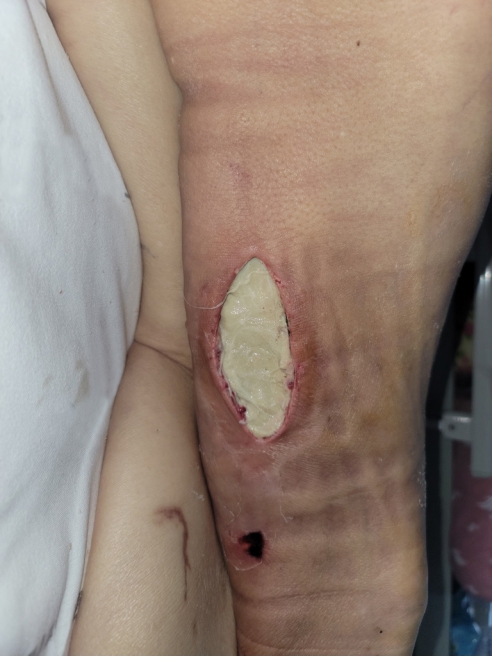
We made a 10-cm incision on the right thigh to drain the abscess (Fig. 6). Four days later, an additional incision was made in the right ischial area to expose the fistula tract, drain the abscess, and remove necrotic tissue. It was confirmed that the fistula tract was connected to the sacral area pressure ulcer (Fig. 7). One week later, the entire posterior aspect of the right thigh was incised to remove necrotic tissue and to confirm its connection to the fistula tract (Fig. 8). Two weeks later, additional debridement was performed on the necrotic tissue in the sacral area and right thigh. From the day of admission until the last debridement, povidone-iodine-soaked gauze dressings were applied 2 to 3 times a day. After the last debridement, during which most of the necrotic tissue was removed, negative pressure wound therapy (NPWT) was initiated and changed 3 to 4 times a week, reducing undermined areas and promoting the growth of granulation tissue (Fig. 9).
During the hospitalization period, follow-up laboratory tests including WBC counts and CRP levels were conducted, with each parameter gradually returning to normal levels. After the removal of the sacral area abscess and necrotic tissue, the WBC count remained within the range of 10,000 to 13,000/μL, and the CRP decreased to 150.3 mg/L. Following the removal of the thigh abscess and necrotic tissue, the CRP decreased to 19 mg/L. Antibiotics were continuously adjusted based on culture and antibiotic susceptibility test results. Regarding patient positioning, the patient had limited cooperation and often lay in the position of his choice. Therefore, the patient was continuously reminded to change position every 2 hours, and efforts were made to ensure he changed positions as frequently as possible.
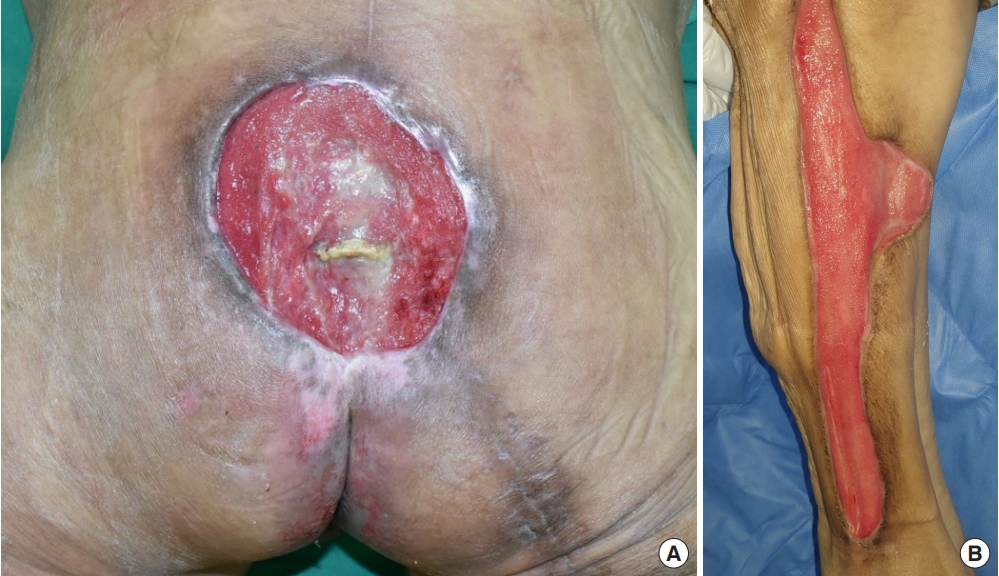
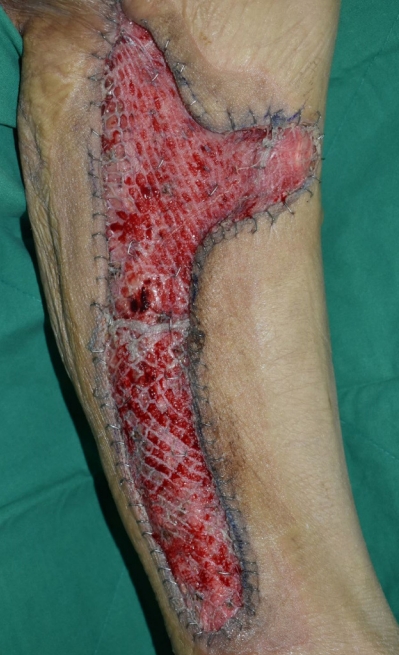
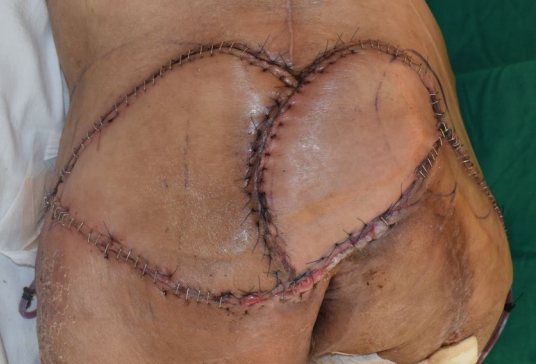
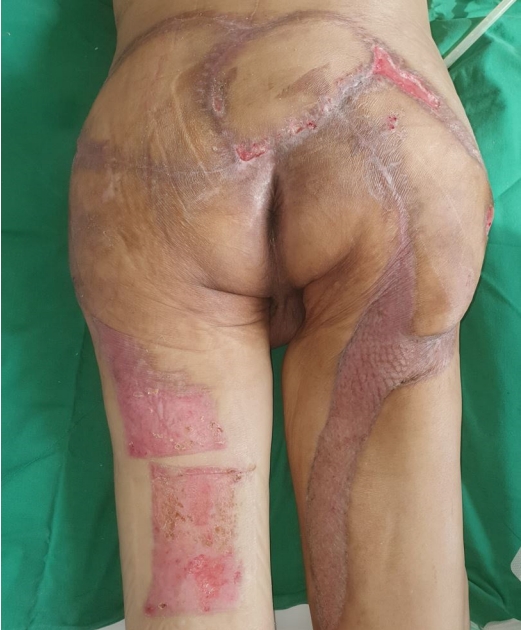
After 3 and a half months of admission, the necrotic tissue was completely removed, leaving a well-prepared wound bed, and reconstructive surgery was performed. To reconstruct the defect in the right posterior thigh, a split-thickness skin graft was harvested from the contralateral thigh and NPWT was applied (Fig. 10). For sacral pressure ulcer reconstruction, a V-Y advancement flap was planned for implementation on the left side and a superior gluteal artery perforator (SGAP) flap on the right side. Doppler examination confirmed adequate blood flow in the SGAPs at the one-third point along a line connecting the posterior superior iliac spine and the greater trochanter of the femur. Thus, both the V-Y advancement and SGAP flaps were applied simultaneously, resulting in complete coverage of the pressure ulcer (Fig. 11). Following the surgery, we made an effort to keep the patient in the prone position as much as possible and changed positions every 2 hours, avoiding the supine position. Five days after surgery, antibiotics were discontinued as there were no signs of inflammation. Two weeks after surgery, once all Hemovac drains were removed and the flap was determined stable, the patient was kept in the supine position for over 4 hours at a time to allow for adequate compression of the flap. Two months after the flap surgery, the wound showed gradual improvement with no signs of deterioration or suspected recurrence, leading to a transfer to another hospital in stable condition (Fig. 12).
Discussion
Pressure ulcers occur when prolonged pressure is applied to the protruding areas of the body, most commonly the sacral area. Continuous pressure on these body parts reduces blood flow, leading to a lack of oxygen and nutrient supply to the tissues that results in tissue damage and ischemic necrosis [3]. Chronic non-healing or worsening pressure ulcers should be considered for potential complications, such as infection, which often requires long-term systemic antibiotic therapy. Bacterial biofilms and the constant release of waste products inhibit healing [4]. As infections progress, life-threatening complications, such as abscess formation, osteomyelitis, and sepsis can occur. In such cases, long-term antibiotic therapy along with surgical interventions, such as debridement, incision and drainage may be necessary.
An abscess is a localized collection of purulent discharge that occurs in response to an infection, and is primarily caused by a microbial infection. Breakdown of the skin barrier due to pressure ulcers can facilitate the invasion of microorganisms, particularly in patients with risk factors, such as advanced age and diabetes [5]. Thigh abscesses can have various clinical presentations, leading to delays in diagnosis and treatment that potentially result in necrotizing fasciitis and a worse prognosis [6]. Diagnosing such conditions before they progress is crucial because they can be successfully treated with incision and drainage, along with appropriate antibiotic administration.
In cases where a pressure ulcer is accompanied by an abscess, an accurate preoperative assessment of the abscess is essential. Sometimes the abscess may be more widespread in the surgical field than anticipated, making complete removal challenging and potentially leading to delayed healing and the need for additional surgeries. Therefore, if there is a possibility of infection, signs of inflammation, such as fever, redness, pain, and the presence of purulent discharge should be investigated [7]. If a physical examination raises the suspicion of an abscess, a preoperative MRI is crucial to confirm its presence, depth, and extent. However, in cases where no specific symptoms are clinically evident, the possibility of an abscess can be overlooked.
Thigh abscesses can arise from primary infections of the hamstring muscles or, although less common, from secondary infections that spread from adjacent structures. Infections originating from the abdomen and pelvis can anatomically involve the nearby thigh muscles [8]. In the case of this patient, he had impaired sensation in his lower extremities due to spinal cord injury, which prevented him from feeling or complaining of pain. No other prominent clinical symptoms were specifically observed in the right thigh. Although there was some swelling, it did not raise suspicion of compartment syndrome to a significant degree. As evident from the MRI findings, inflammation had infiltrated the hamstring muscle, and considering the thigh was soft and supple upon palpation, the possibility of necrotizing fasciitis also seemed low. The E. faecium and E. coli yielded from the wound culture conducted on the pressure ulcer at the time of admission were also isolated from the aspiration culture of the right thigh abscess. Furthermore, during surgery, it was confirmed that the pressure ulcer in the sacral area and the right thigh abscess were interconnected through a fistulous tract in the ischial area. Therefore, in this case, it is highly likely that the infection progressed gradually towards the thigh from the sacral area due to coccygeal osteomyelitis and precoccygeal/posterior sacral soft tissue abscesses.
This case demonstrates the extension of infection from a sacral pressure ulcer to the posterior thigh. It highlights the potential for inflammation to spread to other areas in patients with severe pressure ulcers. Contrast enhanced MRI was helpful for diagnosis, enabling successful treatment with long-term antibiotic therapy, incision and drainage, debridement, and reconstruction. Thus, in patients with severe pressure ulcers and suspected abscesses, physicians should consider the possibility of the extension of abscesses to other areas during diagnosis and treatment.