Introduction
For large soft tissue defects resulting from traumatic injuries, oncologic resections, burns, and pressure injuries, there are several reconstruction options available, including split-thickness skin grafts (STSGs), full-thickness skin grafts (FTSGs), tissue expanders, and various types of flaps. When deciding on the reconstruction method and timing, several factors must be considered, such as the size of the soft tissue defect, the composition of the defect, surgical options, the type of defect, and the surgeon’s preference. In particular, when the soft tissue defect is extensive, the available donor sites on the body are often limited. In cases of large soft tissue defects, staged-reconstruction procedures and multiple donor areas for reconstruction may be necessary. However, using multiple donor sites can lead to several donor-related issues, such as functional deficits at the donor site, multiple scars, hypertrophic or painful scarring, and undesirable pigmentation [1-3]. Donor site morbidity can cause functional and psychosocial problems in addition to cosmetic issues [2]. Therefore, it is essential to consider the limited available donor sites on the body and how surgeons can minimize donor site morbidity.
In our cases involving extensive tissue defects, we attempted staged reconstruction. The first step involved reconstruction with an STSG, followed by reconstruction using an anterolateral thigh (ALT) free flap from the previous STSG donor area. We present our staged-reconstruction technique for large soft tissue defects, aiming to evaluate the efficacy and safety of these methods, as well as identify any potential issues. We have utilized this approach in three lower extremity reconstruction cases. The patients provided written informed consent for the publication of this case report. The study was approved by the Institutional Review Board of Inha University Hospital (2021-03-025-002).
Case
Case 1
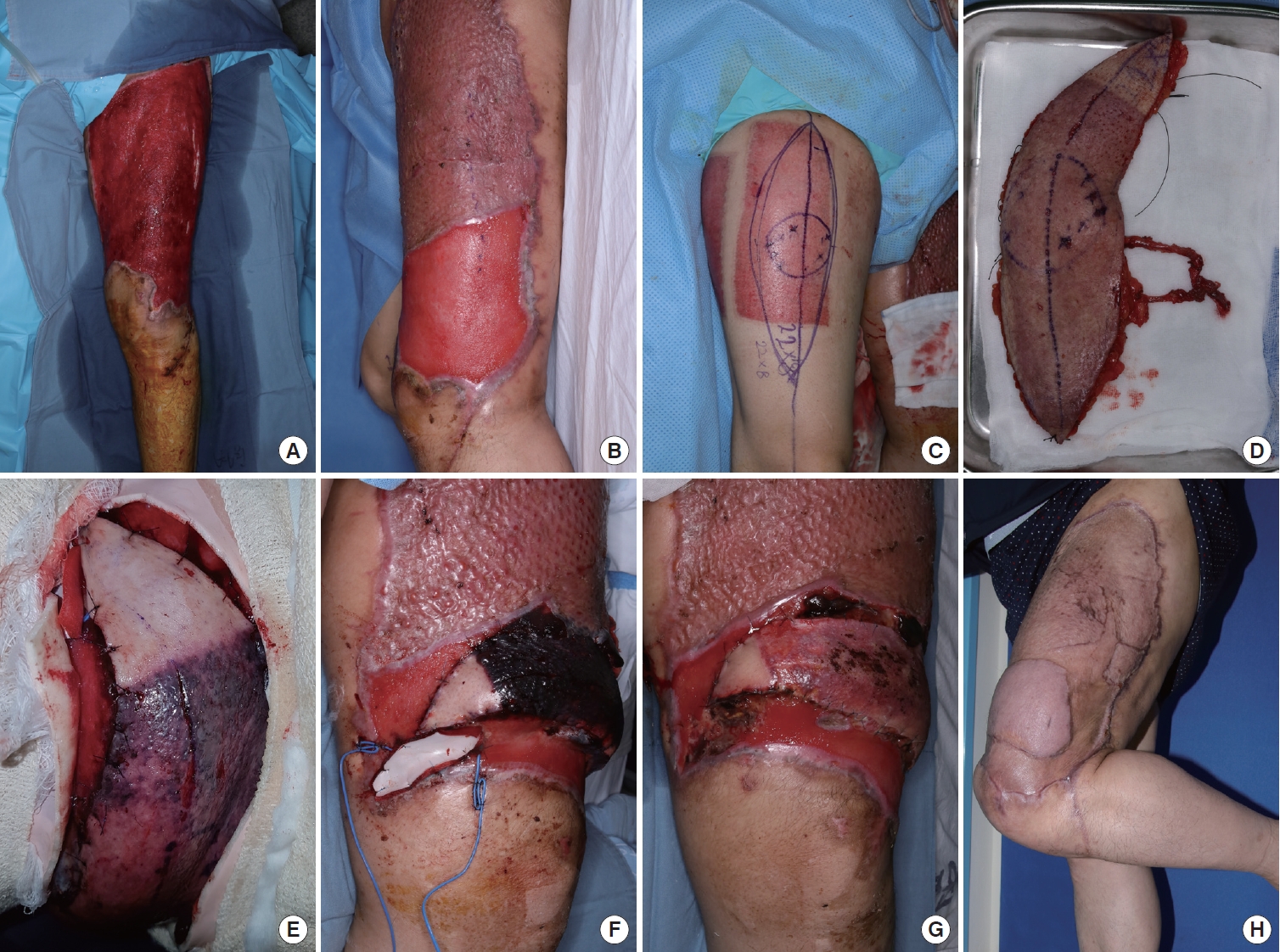
A 48-year-old man sustained a degloving injury to his left thigh and knee in a traffic accident. The patient received aseptic wound dressing for approximately 1 month. The defect measured about 37×32 cm, with no exposure of other deep structures such as bone and ligaments (Fig. 1A). Since the soft tissue defect involved the knee joint, a STSG was planned only for the area proximal to the joint, with an ALT free flap to be performed later to restore range of motion. We also considered performing an STSG and an ALT free flap simultaneously. However, harvesting an STSG from the ALT and elevating the ALT free flap from the same donor site would have made postoperative management difficult. Additionally, the patient’s autologous tissue donor site options were limited. Therefore, we opted for a stepwise approach, first performing an STSG to reduce the size of the defect. We initially performed the STSG using the right thigh as a donor site to cover the defect in the left thigh (Fig. 1B). After 1 month, the remaining soft tissue defect around the knee measured approximately 6×20 cm and was reconstructed with an ALT fasciocutaneous free flap (8×22 cm) from the right thigh, which had previously been used as the STSG donor site (Fig. 1C and D). Immediately after the ALT flap, the skin color appeared pink. However, after 4 hours, the skin color changed to purple (Fig. 1E). The color of the previous STSG donor site on the flap was darkening, while the color of the normal skin on the flap remained pink (Fig. 1F). Handheld Doppler ultrasonography, the pinprick test, and the flap temperature were all normal. After 7 days, the color of the previous STSG donor site on the flap gradually returned to pink. One month after the flap, it had completely survived. With an additional STSG, the wound was fully closed (Fig. 1G and H).
Case 2
A 24-year-old man sustained a crushing injury to his right lower extremity in a traffic accident (Fig. 2A). He underwent debridement, and myorrhaphy was performed on the damaged right tibialis anterior, peroneus longus, and extensor digitorum longus muscles. Negative-pressure wound therapy was applied for approximately 1 month. The soft tissue defect was roughly half the size of the right lower extremity and involved bone exposure of the anterior tibia. In our department, we performed a thoracodorsal artery perforator flap for the exposed tibia (Fig. 2B). After 1 month, the remaining 20×60 cm soft tissue defects were covered with a 1:1.5 meshed STSG from his left thigh (Fig. 2C). Seven months later, the patient complained of limited knee motion. We attempted to reconstruct a free flap for contracture release of the right knee. After releasing the contracted scar on the suprapatellar area, an ALT flap was harvested from the previous STSG donor site. The flap measured 19×8 cm, and the pedicle was located eccentrically at the proximal end of the flap (Fig. 2D and E). The color of the proximal area of the flap was pink, but the distal area, including the previous STSG, appeared dark. Due to the eccentric location of the pedicle, we believed that the distal area of the flap might be situated far from the vascular territories (Fig. 2F and G). On postoperative day 20, after debridement of the partial necrosis, an additional STSG was performed. After 6 months, the patient’s knee flexion improved to 90° (Fig. 2H).
Discussion
Reconstructing large soft tissue defects presents a significant challenge for surgeons, who often rely on autologous tissue, such as skin grafts and free tissue transfers. When addressing soft tissue defects, it is necessary to borrow healthy tissue from other areas of the body for the reconstruction process. Techniques such as STSGs, FTSGs, local flaps, distant flaps, and free flaps can result in donor site morbidity. Factors such as functional deficits, delayed wound healing, wound infections, undesirable pigmentation, hypertrophic scarring, and painful scars must be considered in terms of psychosocial and aesthetic outcomes following reconstruction [1-3]. Another issue to contend with is the limited availability of suitable donor sites.
We considered the idea of using the same donor site twice to reduce donor morbidity in cases with limited donor availability. Consequently, we attempted to harvest autologous tissue twice from the same donor site to minimize donor morbidity by obtaining a flap from a previously used STSG site. The lateral thigh serves as a useful donor site for STSGs due to its easy accessibility and relatively concealed anatomical location. Additionally, the ALT flap is also situated in the same area [4]. There have been a few reports on STSG donor sites from previously harvested free flap tissues [5-7]. However, there have been no reports on STSG donor sites from a predicted ALT free flap, as we have done. We believed that this approach would be a safe procedure to enhance tissue utilization efficiency.
In case 1, we unexpectedly observed a color change in the partial flap, along with difficulty in subjective flap monitoring. However, other conventional monitoring techniques, such as temperature, handheld Doppler, and pinprick test, were normal. Additionally, the color of the normal skin within the flap consistently maintained a pink hue. Although it was challenging to assess flap perfusion, we opted for observation without exploration [8-10]. The color of the entire flap improved after 7 days, ultimately resulting in successful flap survival.
The subpapillary plexus is mainly composed of postcapillary venules and arteriole capillaries [11]. It supplies blood to the epidermis through the papillary loop located inside each upper projection of the dermal papillae. When an STSG is performed, the papillary loop and papillary plexus may be damaged if the graft is harvested at a thickness of 0.008 to 0.010 inches. The STSG donor site then undergoes a healing process, taking on characteristics of hypervascularity and resulting in an erythematous appearance. Erythema can be considered to reflect a higher O2 demand due to new skin production. Erythema persists for several months, and after about a year, the blood vessels regress through a remodeling process, eventually becoming similar in color to the surrounding skin [12]. Following the free flap procedure, the decrease in blood flow led to hypoxic damage in the area with high O2 demand, which is believed to have caused necrosis of the epidermis. It is also thought that venous congestion occurred as blood circulation decreased immediately after the free flap. However, we believe that the hypoxic damage ultimately resulted in the release of several cytokines from the abundant papillary plexus, leading to continuous angiogenesis, which promoted the production of new epidermis and color recovery. Additionally, as the blood flow of the free flap increases over time, venous congestion resolves, and the color is restored [13].
The safe time to harvest ALT flaps after STSG is unclear due to the limited number of cases. In case 1, the ALT flap was harvested 30 days after STSG, and a color change in the partial flap was observed. In patients 1 and 2, no range of motion restriction was observed due to contracture caused by scar formation of the epidermis after performing the ALT free flap from the area used as a donor for the skin graft. This is because the skin graft donor site gradually recovers and becomes similar to the surrounding skin from the 6th week after surgery [12].
In our cases, we utilized soft tissue from the ALT as the donor site twice, thereby doubling the amount typically used. We performed an STSG first, followed by flap elevation at the same donor site. However, flap elevation could be performed first, and the remaining defect could then be covered by harvesting an STSG from the previously transferred flap tissue. This reconstructive approach can also be employed using tissue from the back, specifically the thoracodorsal artery perforator flap. The drawback of this method is the need for multiple stages of reconstruction and the time required for the STSG donor site to heal.
We present two cases of lower extremity reconstruction utilizing autologous tissues (STSG and a free flap) from the same donor to enhance the efficiency of tissue usage. In all instances, successful reconstruction was accomplished while minimizing scarring.