 |
 |

|
| Aims and Scope |
| About the Journal |
| About the Society (KWMS) |
| Editorial Board |
| Open Access |
| Principles of Transparency and Best Practice |
| Editorial Office |
| Subscriptions |


|
| Aims and Scope |
| About the Journal |
| About the Society (KWMS) |
| Editorial Board |
| Open Access |
| Principles of Transparency and Best Practice |
| Editorial Office |
| Subscriptions |

AbstractBackground Diabetic foot wounds are usually chronic and prone to infection. As chronic wounds can induce various malignancies, and malignancies can sometimes present as chronic wounds, the accurate diagnosis of concomitant skin malignancies requires careful attention. We report eight cases of skin malignancies presented with diabetic foot wounds.
Methods Patients who were diagnosed with skin cancer after receiving diabetic foot management from January 2008 to October 2017 at our diabetic foot clinic were selected for this retrospective study. Using an electronic chart review, patient data including age, sex, height, weight, wound characteristics, related systemic diseases, biopsy results, and applied treatments were collected.
IntroductionDiabetic foot wounds are a devastating complication of diabetes, with a 5-year mortality rate of 44% [1], and they significantly affect patients’ quality of life. The pathophysiology of diabetic foot ulcers includes diabetic neuropathy and angiopathy. Diabetic neuropathy can interfere with the patient’s ability to detect the development or recurrence of pressure ulcers on the plantar aspect of the metatarsal heads or heel [2], while diabetic angiopathy causes ischemic necrosis of the toe tips and impedes spontaneous wound healing [3]. Those characteristics of diabetic wounds often make them become chronic and recurrent.
Previous studies have reported that chronic wounds can lead to various malignancies, including squamous cell carcinoma, basal cell carcinoma, Kaposi’s sarcoma, melanoma, and lymphoma [4-12]. Squamous cell carcinoma, which develops in wounds left by chronic osteomyelitis, is a familiar pathology for orthopedic surgeons. However, the relationship between diabetic foot wounds and skin malignancies has not been sufficiently investigated by clinicians, and misdiagnosis or delayed treatment is a risk [9-11]. In addition, malignancy may present as chronic wounds. As diabetic foot wounds are usually chronic and prone to infection, careful observation is required to accurately diagnose concomitant skin malignancies. In this study, we report with a review of literature eight cases of skin malignancies which presented with diabetic foot wounds.
MethodsAfter obtaining approval by the Institutional Review Board of Asan Medical Center (IRB No. S2022-1891-0005), a retrospective review was performed for patients who had diabetic foot with concomitant skin malignancies. Among the patients who received diabetic foot management between January 2008 and January 2023 in the diabetic foot clinic, patients who were also diagnosed with skin cancer were selected for the retrospective study. Using an electronic chart review, patient data such as age, sex, height, weight, wound characteristics, related systemic diseases, biopsy, and treatment were collected. The patients provided written consent for the use of their photographs.
ResultsA total of eight patients who had diabetic foot with concomitant skin malignancies were enrolled in a retrospective cohort study.
Case 1A 71-year-old man presented with a 2-year history of a plantar wound under the left fifth meta-tarsal head. The patient was on medication for hypertension, diabetes mellitus, and a cerebrovascular accident (CVA). His left foot and ankle exhibited an equinovarus deformity caused by the CVA 20 years earlier, and the patient reported experiencing recurrent callosity under the fifth metatarsal head. The wound, devoid of clinical signs of wound infection, was pigmented and measured 1.5×3.0 cm (Fig. 1). The blood sugar test (BST) revealed results of 100–196 mg/dL, and the ankle-brachial index (ABI) of the right and left legs were 1.05 and 0.61, respectively. Quantitative sensory testing revealed no signs of diabetic neuropathy. A punch biopsy was performed, and the pathological findings were consistent with malignant melanoma. No metastases were detected on either whole-body positron emission tomography (PET) or chest, abdominal, and pelvic computed tomography (CT). The malignant melanoma was removed using wide lo-cal excision, and the defect was repaired using a skin graft and percutaneous angioplasty (Fig. 1B).
Case 2A 68-year-old man presented with an 8-month history of a skin defect on the tip of his left fifth toe, which had initially been dark in color (Fig. 2A). The patient also had a history of hypertension, diabetes mellitus, and hyperlipidemia. Wound dressing was initially performed because the wound exhibited no signs of infection and digital circulation appeared good. However, the wound appeared slightly aggravated after 1 month of conservative treatment. Laboratory testing revealed a BST result of 222 mg/dL, a glycated hemoglobin (HbA1c) level of 8.9%, a C-reactive protein (CRP) level of 0.51 mg/dL, and ABI values of 1.18 (right) and 1.21 (left). While the patient’s digital sensory function was intact, a foot X-ray revealed resorption of the distal phalanx of the left fifth toe (Fig. 2B). As the clinician suspected that the concomitant chronic osteomyelitis of the distal phalanx might impede wound healing, a biopsy was performed with wound debridement and minimal bone resection. Pathological examination revealed poorly dif-ferentiated squamous cell carcinoma with chronic inflammation and bone invasion, but no evidence of metastasis. A toe amputation was performed.
Case 3A 75-year-old man presented with a 1-year history of a persistent wound on the nail bed of the right hallux (Fig. 3). The toenail had been extracted spontaneously. Though the patient had developed a diabetic foot wound 3 years earlier after wearing tight shoes, the wound had subsequently healed. Clinical and laboratory examinations revealed no signs of infection, with an initial BST result of 230 mg/dL, an HbA1c level of 7.6%, and ABI values of 1.34 (right) and 1.31 (left). A foot X-ray revealed no signs of bone erosion. The patient reported slightly decreased sensory function, which was not quantitatively tested. A punch biopsy revealed poorly differen-tiated malignant melanoma. A metastatic workup with whole-body PET revealed multiple hypermetabolic lymph nodes at the right inguinal, right external iliac chain, mediastinal pre-vascular, right paratracheal, and left supraclavicular fossa nodes. The patient is being treated using palliative radiation therapy and chemotherapy.
Case 4A 78-year-old man presented with a 3-month history of a wound and exudate on his left hallux (Fig. 4). The patient was receiving medication because of diabetes mellitus, hypertension, and CVA. Laboratory testing revealed a BST result of 156 mg/dL, an HbA1c level of 6.1%, and a CRP level of 0.04 mg/dL. A foot X-ray found no bony erosion. Malignant melanoma was diagnosed based on the pathological results from a punch biopsy. No metastatic lesions were detected and a transphalangeal amputation was performed. Three months later, an inguinal mass was detected via palpation, and an ultrasonography-guided gun biopsy was performed to confirm the presence of metastatic malignant melanoma. Metastatic lesions were also found in the liver. Chemotherapy would have been difficult due to the patient’s poor general condition and the rapid progress of the disease. The patient received palliative care and ultimately died 3 months after the detection of the metastatic lesions.
Case 5A 77-year-old man presented with a 1-month skin lesion between the left fourth and fifth toes (Fig. 5). The patient was receiving medication because of hypertension, diabetes mellitus, and Parkinson’s disease. The diabetes was well-controlled, and laboratory testing found a BST result of 134 mg/dL and an HbA1c level of 5.7%. There were no clinical signs of infection. A punch biopsy was performed, and the pathological results confirmed the diagnosis of acral lentiginous melanoma. A whole-body PET revealed no evidence of metastatic lesions, and a midfoot amputation was performed.
Case 6A 91-year-old man with a 30-year history of diabetes visited our clinic with a 2-year history of a left dorsal foot wound (Fig. 6A). The patient’s medical history only indicated diabetes mellitus and diabetic nephropathy, and his left third and fourth toes had been amputated 20 years earlier because of a diabetic foot wound. At the time of the visit, the wound was hypertrophic and ap-proximately 10×10 cm. Laboratory testing revealed a BST result of 165 mg/dL, an HbA1c level of 6.5%, a blood urea nitrogen level of 30 mg/dL, a serum creatine level of 1.74 mg/dL, and a CRP level of 1.75 mg/dL. Foot radiography showed destruction of the lateral midfoot, which coincided with the location of the wound (Fig. 6B). A punch biopsy confirmed the diagnosis of moderately differentiated squamous cell carcinoma. A whole-body PET detected no metastatic lesions, and the patient subsequently underwent amputation below the knee.
Case 7A 35-year-old man with a 2-year history of diabetes mellitus presented with ulceration and an eschar wound on the right foot plantar side of the hallux at the metatarsophalangeal level. The wound started from a bulla formation after walking for a long time, 6 weeks before his visit to the clinic. The size of the wound was 2.3×1.0 cm (Fig. 7A). At the patient’s first visit, a wound biopsy was performed, showing a fragmented normal epidermis with inflammatory exudate. The wound was assessed as a diabetic foot ulcer and managed conservatively with local debridement. However, the wound did not heal for 8 months, even after serial debridement and proper management. Therefore, an en bloc excision of the ulceration and a full-thickness skin graft was performed. The permanent pathology report of the ulceration showed verrucous squamous cell carcinoma. No metastasis was detected by PET or by a chest, abdominal, and pelvic CT. Under combined operation with the orthopedic department, a wide excision was planned, and the plastic surgery team reconstructed the defect with an anterolateral thigh free flap. The wound then healed without any major complications, and the flap was well-maintained for 18 months (Fig. 7B and C).
Case 8A 79-year-old woman presented with left foot ulceration on the medial malleolus side. She was on medication for hypertension and diabetes. The wound developed 2 years previously and was managed conservatively at the primary clinic. However, 6 months before the referral, the patient visited a local tertiary clinic for further management as the wound was growing and her pain was worsening. After serial debridement, a full-thickness skin graft was performed with a tissue biopsy which indicated squamous cell carcinoma (Fig. 8A). The patient was referred to our clinic for further evaluation and surgical management. PET imaging and an abdominal-pelvic CT suggested a left-sided inguinal lymph node metastasis. A wide excision of the lesion with inguinal lymph node dissection was performed by the orthopedic team, and a soft tissue reconstruction with a contralateral anterolateral thigh free flap was performed by the plastic surgery team. The wound healed without any major complications and the flap was well-maintained for 1 year (Fig. 8B, C).
DiscussionThis report describes our experience with eight cases of foot skin malignancies that involved patients with diabetic foot wounds (Table 1). To the best of our knowledge, this is the largest case series of skin malignancies in diabetic foot patients. As diabetic foot wounds are usually chronic and recurrent, they can lead to skin malignancies. Since it is difficult to determine which lesion developed first, clinicians should be aware of the possibility that these lesions can be malignant.
Previous reports have highlighted possible misdiagnoses of skin malignancies (e.g., malignant melanoma, squamous cell carcinoma, Kaposi sarcoma, and lymphoma) as diabetic foot ulcers in patients with diabetes mellitus [4-6,8-12]. For example, foot wounds in patients with diabetes mellitus are generally considered diabetic foot wounds, and cases in patients with a history of diabetic foot ulcers might be incorrectly diagnosed as recurrent diabetic foot wounds. Furthermore, these patients may visit various practitioners, including general physicians, orthopedic surgeons, plastic surgeons, dermatologists, and specialists in internal, family, and emergency medicine. This sometimes makes it difficult to achieve an early and accurate diagnosis of foot skin malignancies in patients with diabetes mellitus.
The existing evidence is insufficient to conclude whether foot skin malignancies in diabetic patients are primary lesions or if they have developed from chronic wounds. However, several theories support a malignant transformation in chronic wounds, such as exposure to the cytotoxic byproducts of chronic inflammation [13], an impaired mitotic cycle [14], and epidermal implantation resulting in a dermal foreign body reaction [15]. Furthermore, a previous study has suggested that infection could stimulate dormant neoplastic cells to develop into skin malignancy [16]. Another study has proposed that the accumulation of toxins from chronically inflamed cells could induce mutations that lead to malignancy [13]. Moreover, a repetitive deposition of epidermal cells into the dermal layer could cause a foreign body reaction and cellular alteration [13,15].
In this context, nearby mast cells could promote malignant development by releasing neuropeptides and proteases, which alter the normal cutaneous cell and mitotic cycles, and promote endothelial migration of neoplastic cells by destroying the normal skin structure. In addition, mast cell mediators stimulate the proliferation of dormant malignant cells [17].
The most widely known malignancy related to wound chronicity is squamous cell carcinoma, also known as Marjolin ulcer. However, studies show that chronic ulceration is related not only to squamous cell carcinoma, but also to basal cell carcinoma, leiomyosarcoma, and occasionally, melanoma [18-20]. Accordingly, even if the sequence of events might be unclear, physicians should suspect those kinds of malignancies in unusual diabetic ulcer cases.
In 2019, Linkeviciute-Ulinskiene et al. [21] described the malignancy risk pattern among type-2 diabetes mellitus (T2DM) patients throughout the entire population of Lithuania. Among males with T2DM, a significantly increased risk was found for cancer of the liver (standardized incidence ratio [SIR], 2.11; 95% confidence interval [CI], 1.79–2.49), pancreas (SIR, 1.77; 95% CI, 1.57–1.99), kidney (SIR, 1.46; 95% CI, 1.31–1.62), and thyroid (SIR, 1.83; 95% CI, 1.32–2.54). Colorectal cancer (SIR, 1.23; 95% CI, 1.14–1.33), skin melanoma (SIR, 1.40; 95% CI, 1.11–1.76), non-melanoma skin cancer (SIR, 1.14; 95% CI, 1.05–1.23), and cancer of the male genital organs (SIR, 1.86; 95% CI, 1.27–2.71) and other endocrine organs (SIR, 1.96; 95% CI, 1.05–3.64) also had significantly elevated risks. As shown in the study, skin melanoma and non-melanoma skin cancer prevalence in T2DM patients significantly increased. Therefore, these skin cancers should be suspected and investigated when ruling out possible malignancies during treatment of diabetic ulcer patients.
Four of the eight cases (Cases 2, 3, 4, and 6) exhibited hypertrophic wounds that appeared to be well vascularized. Therefore, while concomitant infection might impede wound healing, clinicians treating chronic non-healing wounds with well-vascularized beds should also consider the possibility of underlying skin malignancies. Furthermore, a well-vascularized wound at the toe tip (Case 2) is not a typical feature of diabetic foot wounds, as the toe tips are prone to ischemic insult and usually exhibit dark, necrotic, or mummified wounds.
In Case 1, while the dark pigmented wound could easily have led to a melanoma diagnosis, the hemorrhagic crust and overlying hyperkeratotic skin obscured the signs. Furthermore, acral lentiginous melanoma or amelanotic melanoma near the plantar aspect or toenails is misdiagnosed approximately 40% of the time [22]. Metzger et al. [22] retrospectively reviewed cases of plantar melanoma and observed that this misdiagnosis led to an approximately 12-month delay in appropriate treatment, which had adverse effects on tumor thickness (increasing from 1.5 mm to 5.0 mm) and 5-year survival rate (decreased from 68.9% to 15.4%). Fortunately, our case of acral lentiginous melanoma was quickly diagnosed by an experienced dermatologist. Clinicians must stay vigilant against this type of skin malignancy when they are treating patients with diabetic foot wounds.
The increasing prevalence of diabetes mellitus is expected to lead to an increase in the incidence of diabetic foot wounds. The current treatments for diabetic foot wounds involve infec-tion control, circulation recovery, and off-loading, and there are no guidelines for biopsies of diabetic foot wounds based on timing, lesion size, or severity of lesion. Regarding wound management, a 50% reduction in wound area over a 4-week period is generally considered an indicator that the wound is likely to heal within 3 months [23]. The mean duration of diabetic foot ulcers was 12 months in our study and 18 months in other studies. In 2015, Onesti et al. [24] reported that chronic ulcers tended to persist for 9 years before skin malignancy. This indicates that diabetic foot ulcerations generally last shorter than the time it takes to come to a diagnosis of a skin malignancy in chronic ulcers. Therefore, wounds that fail to heal well should be reassessed using tissue biopsy to determine the underlying pathology [25]. We suggest that clini-cians consider reassessing wounds with a biopsy when they are managing patients with diabetic foot wounds.
Conflict of InterestHyunsuk Peter Suh is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported. Fig. 1.Case 1. (A) A 71-year-old man presented with a 2-year history of a plantar wound under the left fifth metatarsal head. Malignant melanoma was arising from a dark pigmented wound under the fifth metatarsal head. (B) A wide excision and skin graft for the defect were performed. 
Fig. 2.Case 2. (A) A 68-year-old man presented with an 8-month history of a skin defect on the tip of his left fifth toe, with a wound that bled profusely. (B) A foot X-ray revealed bony erosion of the distal phalanx of the left fifth toe, which coincided with the location of the wound. The biopsy showed poorly differentiated squamous cell carcinoma. 
Fig. 3.Case 3. A 75-year-old man visited our diabetic foot clinic with a 2-year history of a persistent wound on the nail bed of his right hallux. The toenail had been extracted and the nail bed appears hypertrophic and well vascularized. The pathology results from a punch biopsy revealed poorly differentiated malignant melanoma. 
Fig. 4.Case 4. A 78-year-old man presented with a 3-month history of a wound and exudate on his left first toe. A malignant melanoma diagnosis was confirmed based on the pathological results from a punch biopsy, and a transphalangeal amputation was performed. 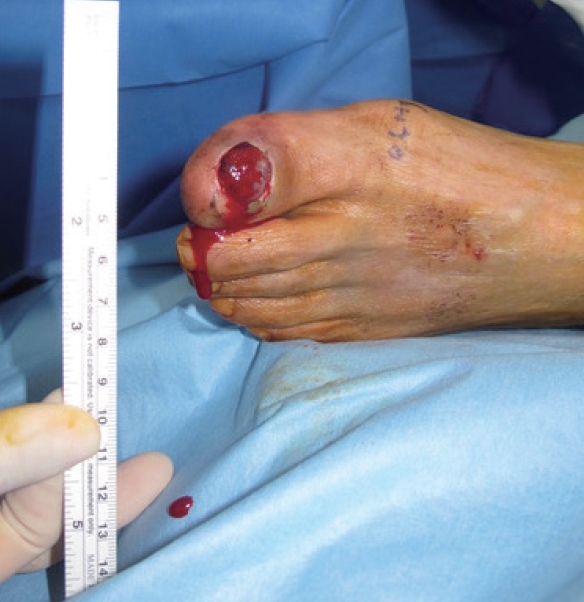
Fig. 5.Case 5. A 77-year-old man visited our clinic with a 1-month history of a skin lesion between the left fourth and fifth toes. The pathological results confirmed the diagnosis of acral len-tiginous melanoma. 
Fig. 6.Case 6. (A) A 91-year-old man with a 30-year history of diabetes visited our clinic with a 2-year history of a left dorsal foot wound. (B) A foot X-ray revealed destruction of the lateral midfoot, which suggested tumor invasion or osteomyelitis. A punch biopsy confirmed moderately differentiated squamous cell carcinoma. 
Fig. 7.Case 7. (A) A 35-year-old man with a 2-year history of diabetes mellitus presented with ulceration and an eschar wound on the right foot plantar side of the hallux at the metatarsophalangeal level. The permanent pathology report of the ulceration showed verrucous squamous cell carcinoma. (B) A wide excision was performed, and the defect was reconstructed with an anterolateral thigh free flap. (C) Postoperative findings after 18 months. 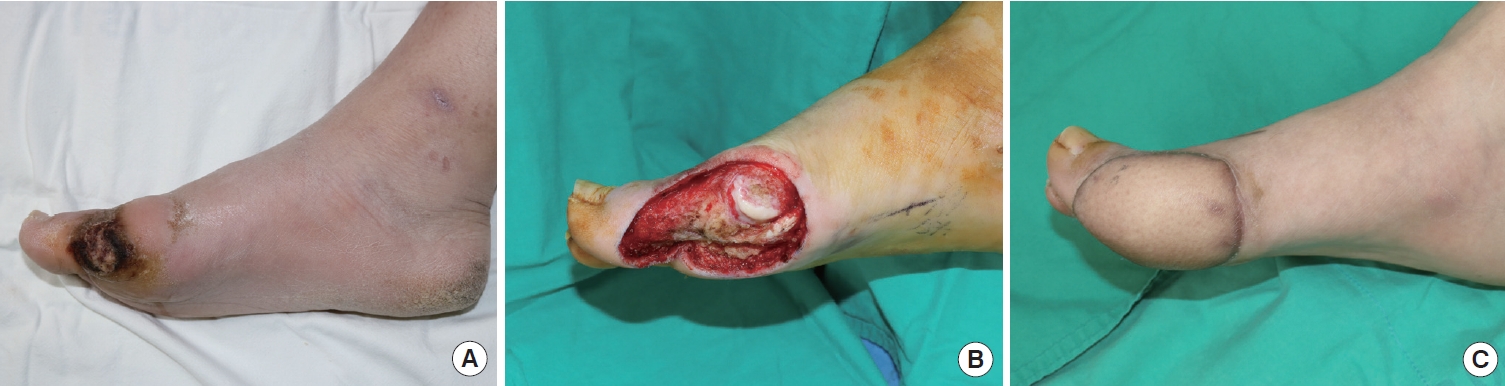
Fig. 8.Case 8. (A) A 79-year-old female was referred from another hospital with biopsy-proven squamous cell carcinoma in left foot ulceration on the medial malleolus side. The lesion was assumed to be diabetic ulceration and was managed conservatively at the primary clinic. (B) A wide excision was performed, and the defect was reconstructed with an anterolateral thigh free flap. (C) Postoperative findings after 1 year. 
Table 1.Overall demographics of the patients References1. Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care 2003;26:491-4.
2. Mantey I, Foster AV, Spencer S, et al. Why do foot ulcers recur in diabetic patients? Diabet Med 1999;16:245-9.
3. Gerassimidis T, Karkos CD, Karamanos D, et al. Current endovascular management of the ischaemic diabetic foot. Hippokratia 2008;12:67-73.
4. Park HC, Kwon HI, Kim HW, et al. A digital squamous cell carcinoma mimicking a diabetic foot ulcer, with early inguinal metastasis and cancer-related lymphedema. Am J Dermatopathol 2016;38:e18-21.
5. Panda S, Khanna S, Singh SK, et al. Squamous cell carcinoma developing in a diabetic foot ulcer. Int J Low Extrem Wounds 2011;10:101-3.
6. Chiao HY, Chang SC, Wang CH, et al. Squamous cell carcinoma arising in a diabetic foot ulcer. Diabetes Res Clin Pract 2014;104:e54-6.
7. Schnirring-Judge M, Belpedio D. Malignant transformation of a chronic venous stasis ulcer to basal cell carcinoma in a diabetic patient: case study and review of the pathophysiology. J Foot Ankle Surg 2010;49:75-9.
8. Caminiti M, Clerici G, Quarantiello A, et al. Kaposi’s sarcoma misdiagnosed as a diabetic plantar foot ulcer. Int J Low Extrem Wounds 2009;8:120-2.
9. Gregson CL, Allain TJ. Amelanotic malignant melanoma disguised as a diabetic foot ulcer. Diabet Med 2004;21:924-7.
10. Gao W, Chen D, Ran X. Malignant melanoma misdiagnosed as diabetic foot ulcer: a case report. Medicine (Baltimore) 2017;96:e7541.
11. Torres T, Rosmaninho A, Caetano M, et al. Malignant melanoma misdiagnosed as a diabetic foot ulcer. Diabet Med 2010;27:1302-3.
12. Iacopi E, Coppelli A, Goretti C, et al. Type 2 diabetic patient with a foot ulcer as initial manifestation of diffuse large B-cell lymphoma: a case report. Diabetes Res Clin Pract 2016;115:130-2.
13. Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer 1975;35:600-5.
14. Giles GG, Marks R, Foley P. Incidence of non-melanocytic skin cancer treated in Australia. Br Med J (Clin Res Ed) 1988;296:13-7.
15. Arons MS, Lynch JB, Lewis SR, et al. Scar tissue carcinoma: I. A clinical study with special reference to burn scar carcinoma. Ann Surg 1965;161:170-88.
16. Gan BS, Colcleugh RG, Scilley CG, et al. Melanoma arising in a chronic (Marjolin’s) ulcer. J Am Acad Dermatol 1995;32:1058-9.
17. Ch’ng S, Wallis RA, Yuan L, et al. Mast cells and cutaneous malignancies. Mod Pathol 2006;19:149-59.
18. Senet P, Combemale P, Debure C, et al. Malignancy and chronic leg ulcers: the value of systematic wound biopsies: a prospective, multicenter, cross-sectional study. Arch Dermatol 2012;148:704-8.
19. Combemale P, Bousquet M, Kanitakis J, et al. Malignant transformation of leg ulcers: a retrospective study of 85 cases. J Eur Acad Dermatol Venereol 2007;21:935-41.
20. Kelahmetoglu O, Cengiz FP, Yildiz P, et al. Melanoma arising in a chronic pressure ulcer. Int Wound J 2019;16:572-3.
21. Linkeviciute-Ulinskiene D, Patasius A, Zabuliene L, et al. Increased risk of site-specific cancer in people with type 2 diabetes: a national cohort study. Int J Environ Res Public Health 2019;17:246.
22. Metzger S, Ellwanger U, Stroebel W, et al. Extent and consequences of physician delay in the diagnosis of acral melanoma. Melanoma Res 1998;8:181-6.
23. Boulton AJ. The diabetic foot: from art to science. The 18th Camillo Golgi lecture. Diabetologia 2004;47:1343-53.
|
|