Introduction
Full-thickness cranial bone defects commonly occur after head trauma or infection; repair by solid cranioplasty using autogenous or alloplastic materials is usually war-ranted for protection and aesthetics [1]. Although cranioplasty with alloplastic bio-materials is less invasive and simpler than autografting, allografting shows a higher rate of complications, especially for patients who have experienced complex trauma or those who undergo repeated surgery or radiation therapy [2]. Of all the complications, implant exposure is the most common complication of cranioplasty, affecting up to 9.6% of patients [3].
This report presents the case of a patient with a recurrent forehead skin defect with mesh exposure who underwent cranioplasty with titanium mesh, following craniectomy to manage intracranial hemorrhage and frontal bone fracture. To treat the skin defect with mesh exposure, two latissimus dorsi (LD) free flaps were applied, along with additional cranioplasty with hydroxyapatite bone cement. The study was ap-proved by the Institutional Review Board of Inje University Ilsan Paik Hospital (IRB No. 2021-03-018) and the patient provided written informed consent for the publication and the use of his images.
Case
The patient was a 32-year-old man without any medical history. He had first presented to the department of neurosurgery when he was 24 years old, in a state of unconsciousness with contusional intracranial hemorrhage accompanying a frontal bone fracture caused by a motorcycle accident. The patient underwent emergent craniectomy of the frontal bone. After 6 months, as his symptoms improved, the patient had cranioplasty with titanium mesh to reconstruct the bony defect. Two years after the cranioplasty, the patient presented to the department of plastic and recon-structive surgery with a skin defect of the forehead. The defect was 2.5 cm in diameter and was accompanied by mesh exposure and signs of infection including leaking pus and redness of skin around the defect (Fig. 1A). Pseudomonas aeruginosa was identified from wound cultures at the site of mesh exposure. Extensive adhesion between thinned skin and titanium mesh, greenish pus and necrotic tissues were identified inside the defect. To cover the defect, we decided to apply a LD free flap. Under general anesthesia, an LD flap 8 cm wide and 5 cm long was harvested from the right back.
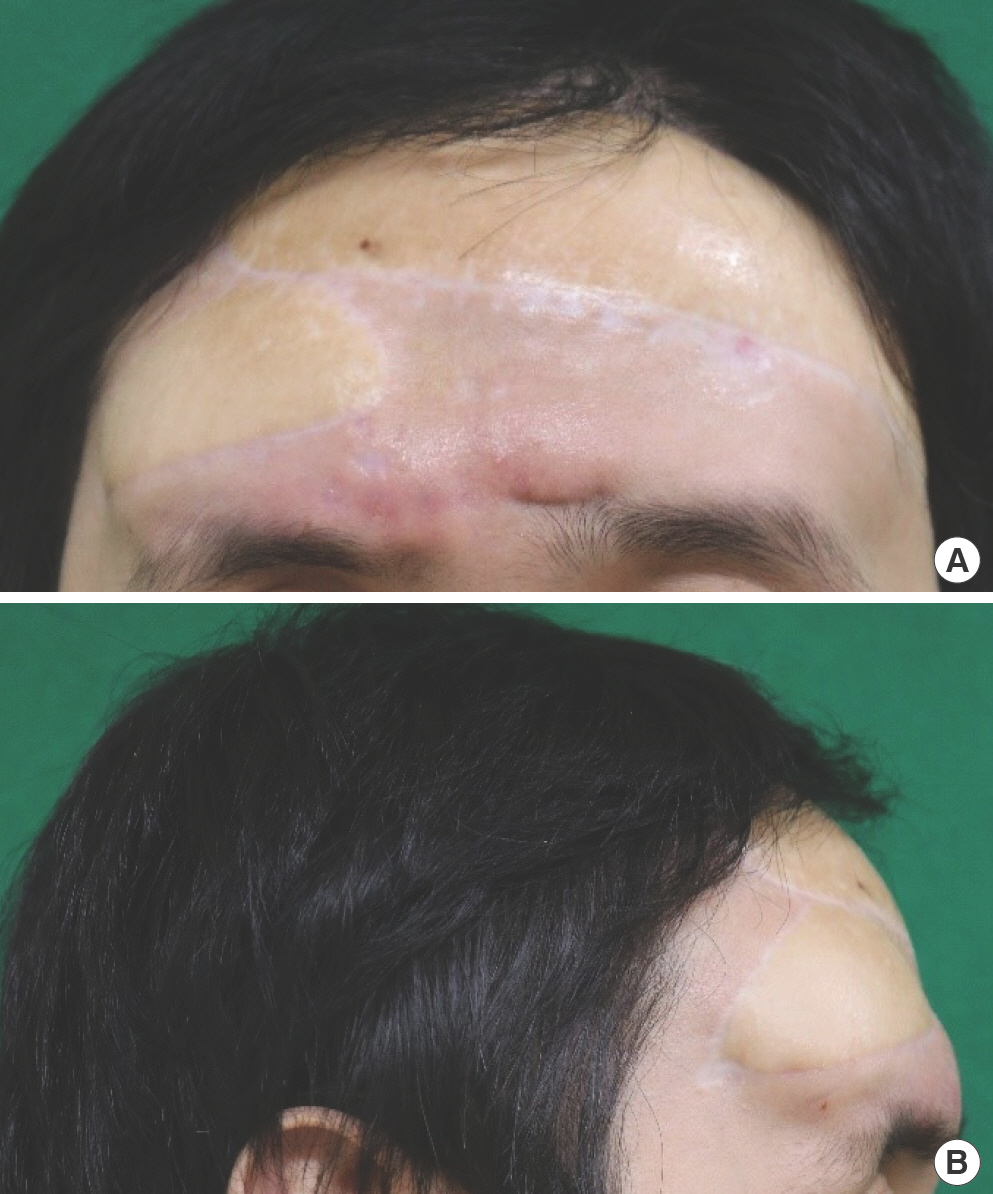
Fig. 1.
Photographs obtained when the first skin defect occurred. (A) The defect was 2.5 cm in diameter and accompanied by mesh exposure. (B) Ten months after the first latissimus dorsi free flap, a round defect 1 cm in diameter with mesh exposure was observed.
As signs of infection were observed at the site of the defect, the musculocutaneous flap was harvested and placed on the forehead with end-to-end anastomosis to the right superficial temporal artery and vein. Also, intravenous ciprofloxacin was used for 1 month to treat the P. aeruginosa infection. Though we recommended that a neurosurgeon completely remove the mesh before the LD free flap operation since repetitive mesh exposure was expected in other areas where the free flap was not executed, the mesh was not removed because the patient and neurosurgeon expressly wished to retain it to avoid defor-mation of the forehead, and also because additional cranioplasty would be necessary to protect the dura and brain. Ten months after the first surgery, the patient reconsulted our department due to another skin defect of the forehead, 1 cm in diameter above the previous operation site (Fig. 1B). We applied an annexed LD free flap to manage this defect and per-suaded the neurosurgeon to remove the titanium mesh because if the mesh was left in place, the defect was expected to reoccur owing to the thinning of skin overlying the titanium mesh. He accepted our recommendation, and a second LD free flap was applied. However, not only because the neurosurgeon did not want to get rid of the mesh around the right eyebrow to maintain the shape of the supraorbital rim, but also because the pedicle of the first LD free flap limited access and reconstruction of the supraorbital rim, part of the titanium mesh around the right eyebrow was left unremoved. Since the skin on the titanium mesh had already become considerably thinner, we decided to transplant the largest free flap possible, for which an LD flap 17 cm wide and 7 cm long was harvested from the left back. Similar to the previous operation, the flap was placed on the forehead with anastomosis to the left superficial temporal artery and vein with end-to-end anastomosis. While no other complications were noted for the next 6 months, downward repositioning of the flap was observed because the mesh underneath the flap had been removed (Fig. 2). Our department and the department of neurosurgery agreed for a neurosurgeon to perform cranioplasty with a material other than titanium mesh and for a plastic surgeon to perform flap revision. Cranioplasty with hydroxyapatite bone cement and modification of the flap were subsequently performed (Figs. 3–5). One year later, revision surgery with complete removal of mesh remaining in the right eyebrow and reconstruction of the supraorbital rim with hydroxyapatite bone cement was carried out. The result was satisfactory, and the surgical site remained stable with no major complications during the 4-month follow-up period (Fig. 6).
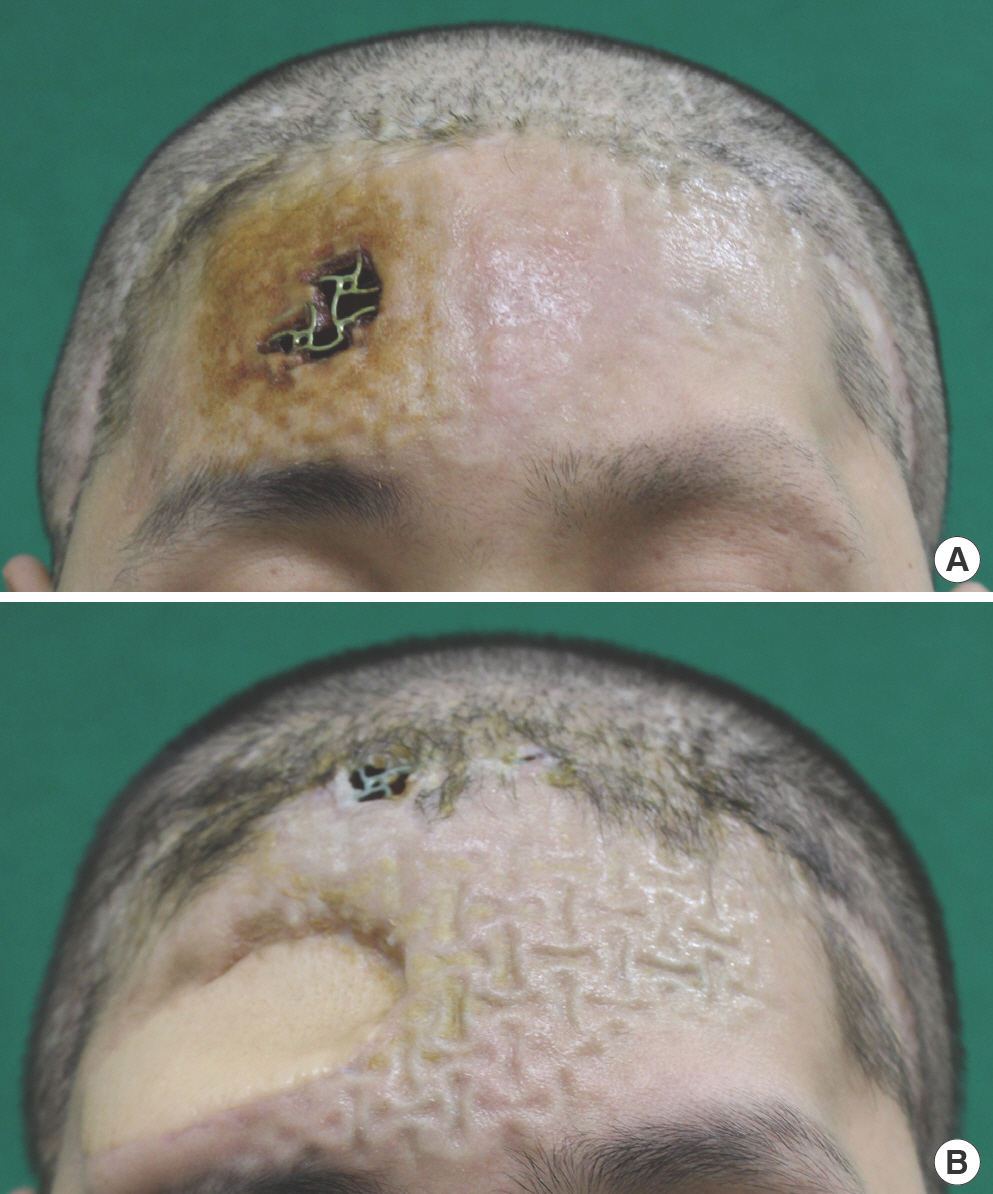
Fig. 2.
Six months after the second latissimus dorsi free flap. (A) Frontal view. (B) Right lateral view. Owing to the absence of mesh under the flap, downward repositioning of the flap was observed.
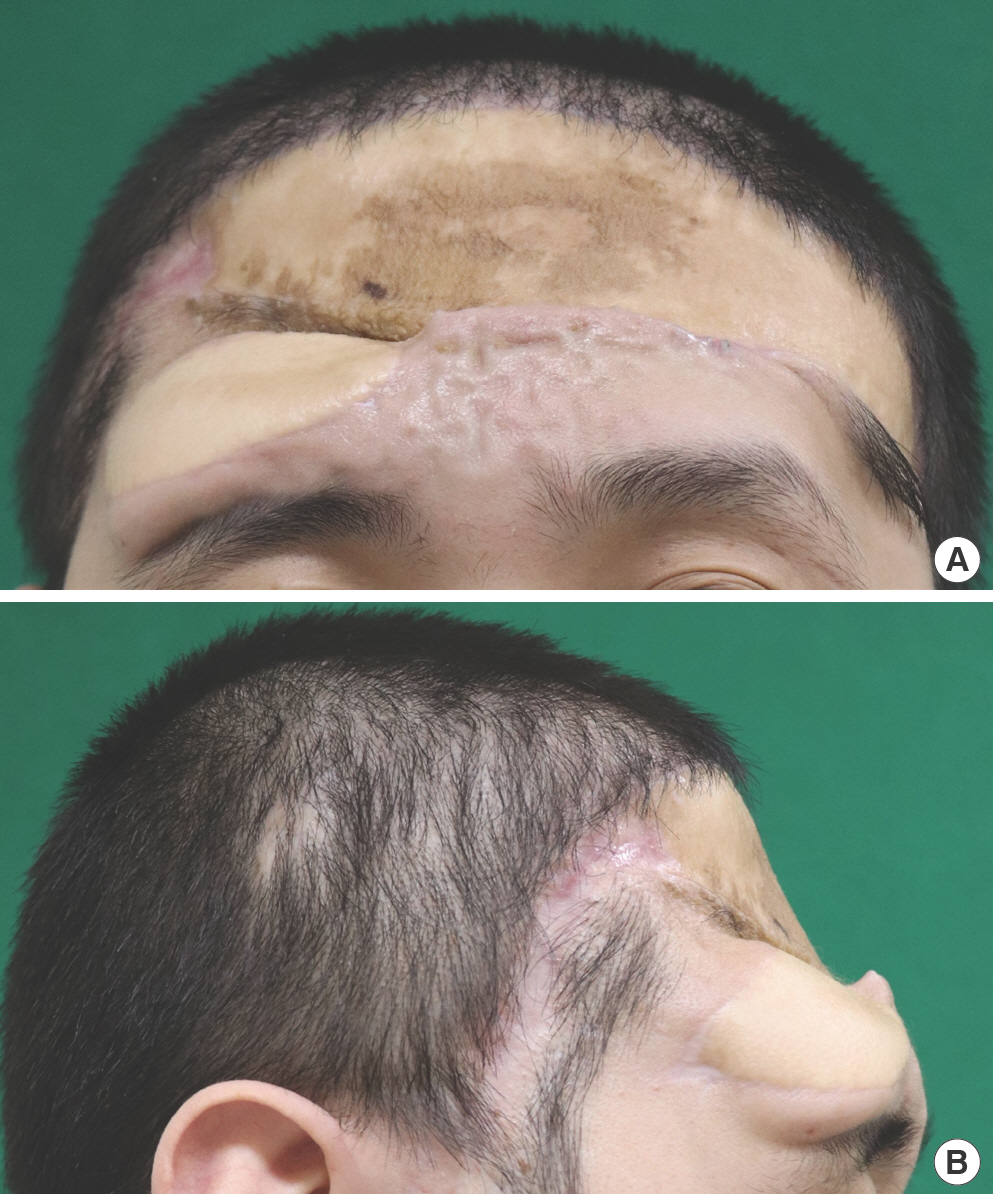
Fig. 3.
Photographs taken after the cranioplasty using hydroxyapatite bone cement. (A) Cranioplasty was executed with hydroxyapatite bone cement. (B) Photograph taken immediately postoperatively. The excessive skin below the second latissimus dorsi flap was excised.
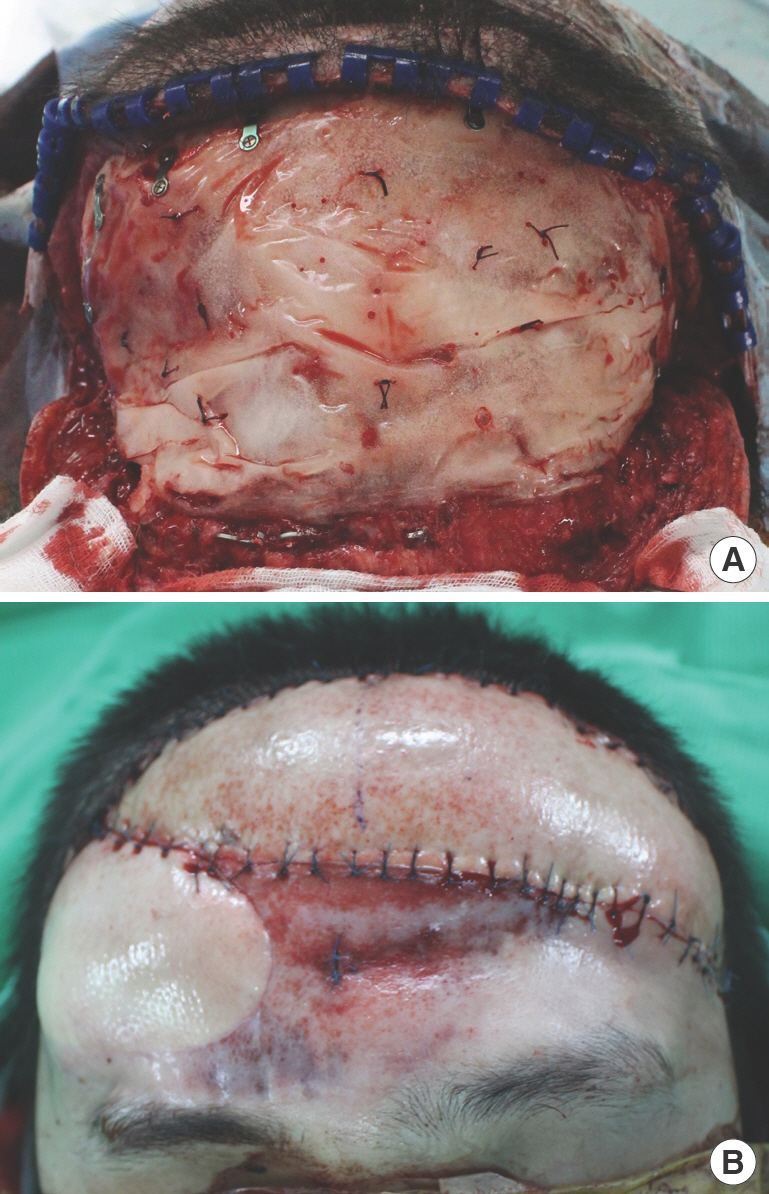
Fig. 4.
Photographs taken after the revisional operation with cranioplasty. (A) Frontal view. (B) Right lateral view. The downward repositioning of the flap improved satisfactorily.
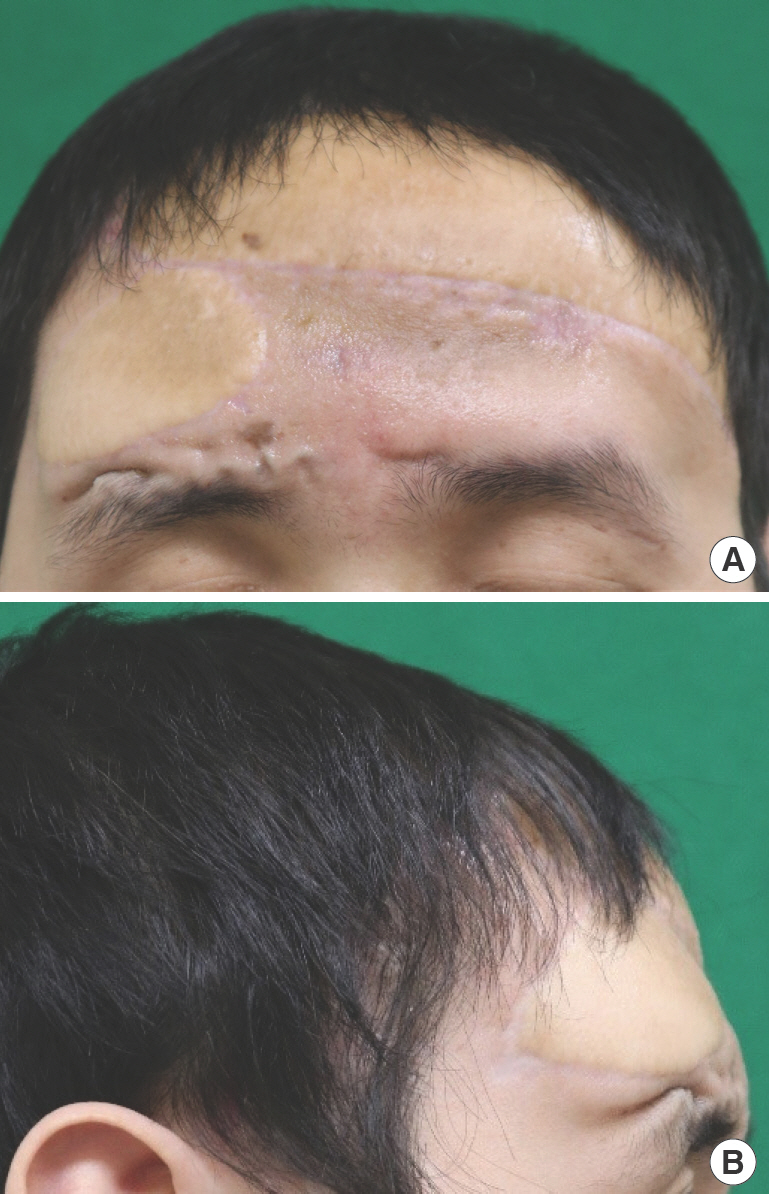
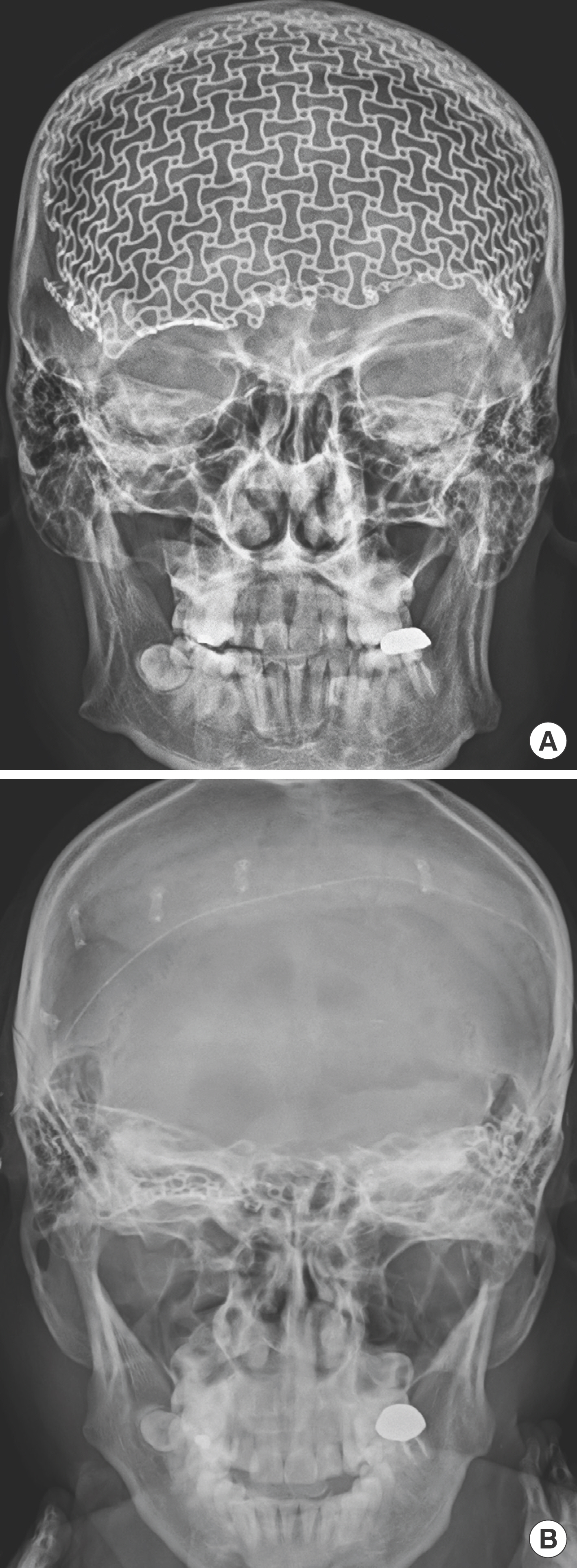
Fig. 5.
Skull X-ray before and after near-total removal of mesh. (A) Skull X-ray taken before the near-total removal of titanium mesh and second latissimus dorsi free flap. (B) Skull X-ray taken after the cranioplasty with hydroxyapatite bone cement. Near-to-tal removal of mesh except for the area of right eyebrow.
Discussion
As cranial bone plays a role in not only protecting vital structures but also maintaining the shape of the head, cranioplasty is a widely used technique to manage skull defects and bony abnormalities. Depending on the material used, cranioplasty can be categorized into the two main categories of biological and synthetic. Although the former has the advantages of a lower rejection rate, higher biogenic compatibility, moldability and ability to integrate with bones, especially in pediatric patients, the latter is commonly used because cranioplasty using synthetic implants reduces the duration of operation and pro-vides better aesthetic results via three-dimensional printing and computer-based customization. Use of titanium mesh cranioplasty is particularly preferred because it prevents aesthetic deformity, decreases the vulnerability of brain tissues, and minimizes the risks and expenses associated with secondary procedures [4].
Meanwhile, there are a number of complications following cranioplasty, such as hematoma, infection and the occurrence of skin defects. When titanium mesh is used, skin defect with implant exposure has been reported in 17.0% of patients [5]. This is presumed to be due to the pressure gradient between the extradural space and atmosphere, similar to the sinking skin flap syndrome, caused by the perforated nature of the titanium mesh [6,7]. Additionally, atrophy and thinning of the scalp overlying the mesh and surgical site infection contribute to the occurrence of soft tissue defects [1,8]. In terms of implant exposure rates, several materials had shown lower rates than titanium. Polyetheretherketone had a clearly lower rate of implant exposure (3%) than titanium, and polymethylmeth-acrylate (PMMA) also had low propensity for implant exposure (2% for hand-molded PMMA, 7% for prefabricated PMMA) [9]. Though the exact implant exposure rate of hydroxyapatite is not known, the rate is expected to be very low as hydroxyapatite implants do not need metal fixation hard-ware unlike other non-titanium implants [9,10].
Standard management of skin defects with titanium mesh exposure involves wound debridement, and removal or ex-change of implants to prevent secondary infection and to achieve suitable aesthetic outcomes [6,11]. Appropriate coverage with skin flaps, such as forehead muscle flaps, temporal is-land flaps and LD free flaps should be performed afterwards [2,12]. However, few studies have described cases of recurrent skin defects from not completely removing the titanium mesh. Among the flap techniques, the LD musculocutaneous free flap is a widely used flap for managing large defects with versa-tility and reliability [12]. As the LD flap contains not only a skin flap but also muscle, it has the potential to control infection of the defect site without severe donor-site morbidity. Additionally, the relatively thick dermal layer of the LD flap pro-vides durability of the flap and is likely to reduce the possibility of re-exposure of the implant.
As our case and previous studies show, titanium mesh underneath the skin leads to a higher rate of soft tissue defects with mesh exposure compared to other materials used for cranioplasty. Therefore, the metal implant should be completely removed to prevent the recurrence of exposure [5,9,10]. In our case, because access to and reconstruction of the supraorbital rim around the right eyebrow of our patient was limited due to the pedicle of the first LD free flap, a small amount of mesh remained, as shown in Fig. 4A. Because this retained mesh was expected to cause another defect, we removed the mesh and reconstructed the supraorbital rim with hydroxyapatite bone cement through an incision below the first flap.
After removal of the metal component, another cranioplasty with different foundational material, such as hydroxyapatite bone cement, as in our case, should be applied to maintain the ideal shape of the forehead and prevent downward repositioning of the overlying skin. Additionally, a valid skin-covering procedure should be chosen in consideration of the condition of the wound and for complete coverage of the metal mesh with a flap of sufficient thickness if the mesh cannot be completely eliminated. From this perspective, we chose to harvest large, durable LD musculocutaneous free flaps to manage the infection and minimize donor site morbidity.
As soft tissue defects are the most common complication after cranioplasty using titanium mesh, and can lead to destructive consequences, appropriate and organized management strategies should be applied. We expect our case study to serve as a lesson when faced with similar conditions. As the exposure rate of titanium mesh is higher than that of other materials used for cranioplasty, and skin defects are highly likely to recur if the titanium is not completely removed, we propose that skin defects with titanium mesh exposure must be treated with complete removal of the involved metal component, followed by cranioplasty using another substance and proper reconstruction of the overlying soft tissue to prevent further recurrence of the skin defect.