Introduction
Cat-scratch disease (CSD) is an infection that usually presents itself as tender lymphadenopathy. Cat-scratch disease is the most commonly recognized manifestation of infection with Bartonella, a Gram-negative rod: Bartonella henselae (most frequent), Bartonella quintana, and Bartonella bacilliformis. These microorganisms isolate from fleas, residing on infected cats, which can contaminate saliva and can then be transmitted to humans via biting and scratching by cats [1].
The bacterial infection usually leads to a regional subacute lymphadenopathy (LAP) in the lymphatic pathways proximal to the region of inoculation.
We report a case of a woman who was a skin therapist suffering axillary lymphadenitis and concomitant non-tuberculous mycobacterial infection due to CSD with no history of cat exposure.
Case
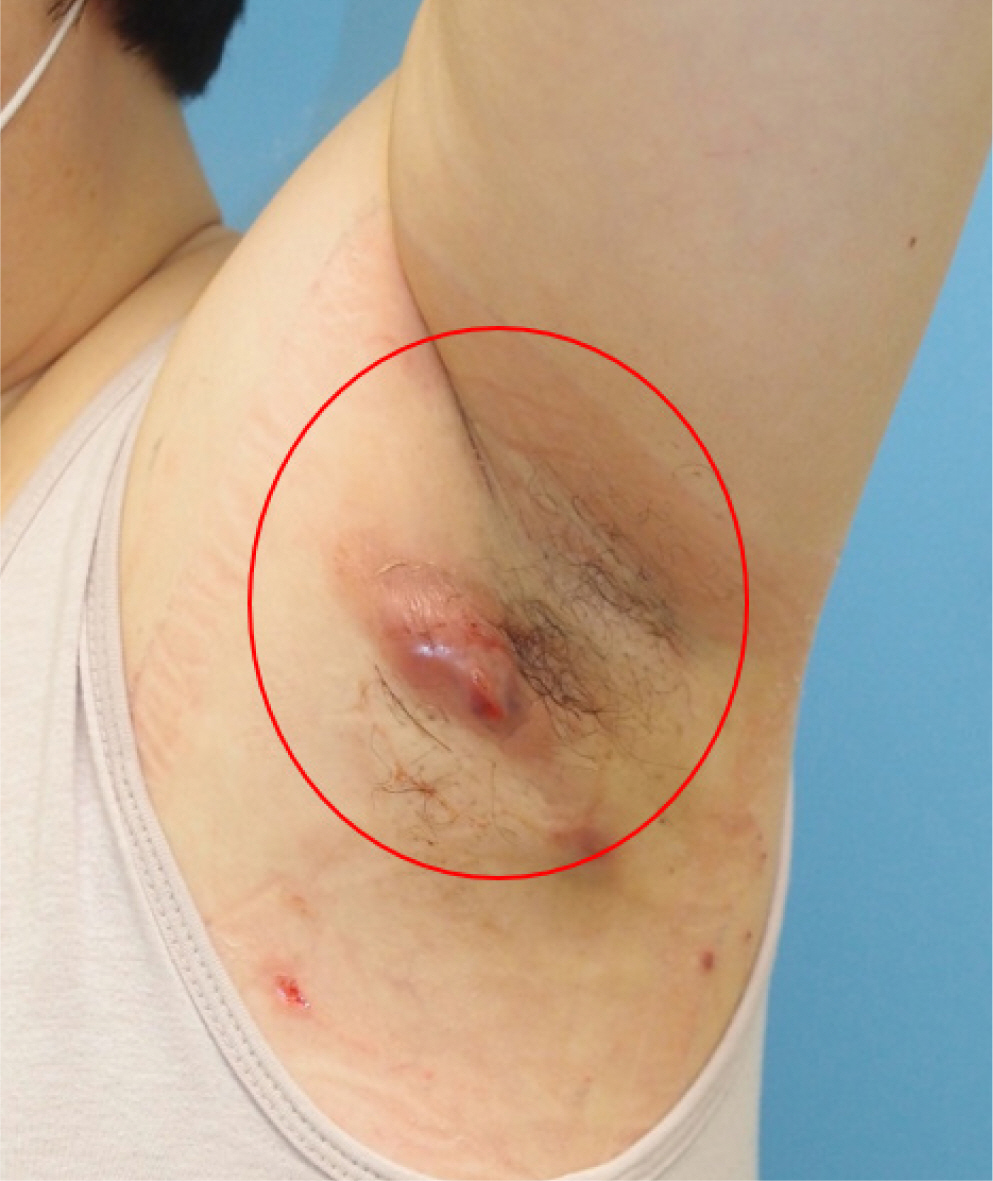
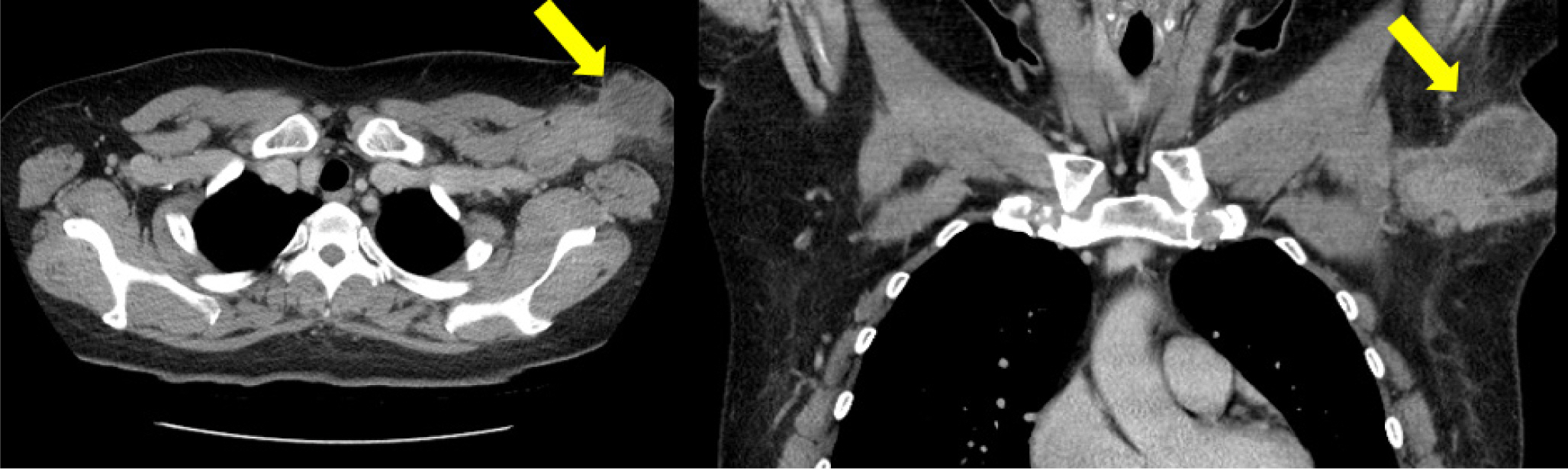
A healthy 42-year-old woman reported with lymphadenopathy on her left axilla which had been occurring for the past 4 months (Fig. 1). On physical examination, we detected a hard and fixed nodule with a diameter of 4.0 cm. A computed tomography scan revealed an enlarged lymph node in left axilla with a necrotic lesion, suggesting abscess formation and lymphadenitis (Fig. 2).
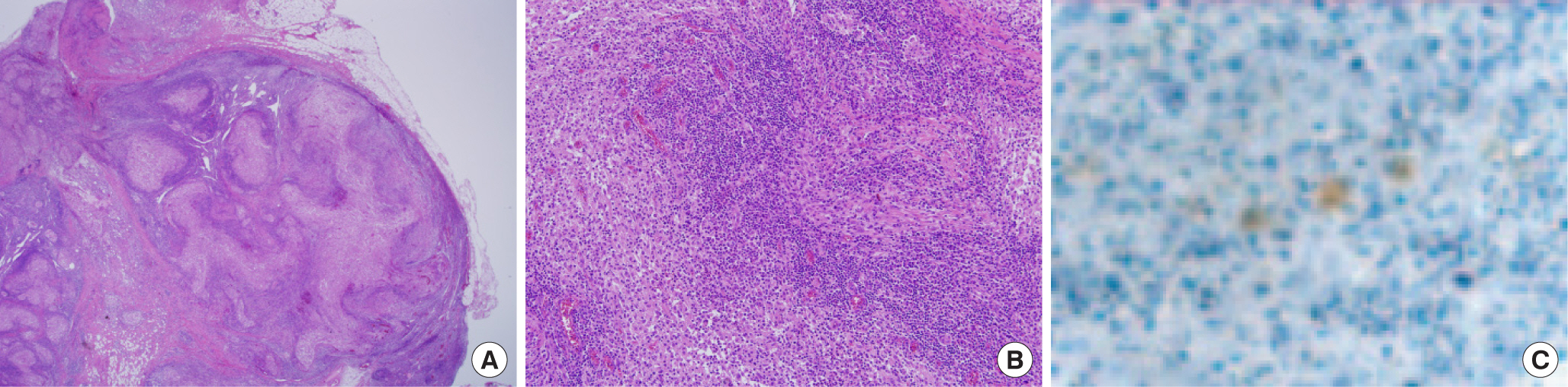
As an initial step, fine-needle aspiration of the lymph node was performed and a necrotizing granulomatous lymphadenitis and tuberculous mycobacterium infection was suspected. The patient underwent surgery, and the enlarged lymph nodes were excised together along with total drainage of the abscess formation (Fig. 3). The histological findings confirmed the granulomatous lymphadenitis and multiple microabscesses were found by using Warthin-Starry silver stain in the lymph node and B. henselae was also found in a form of short rods (Fig. 4). CSD was confirmed despite the patient having no history of cat exposure. The patient probably had indirect contact with cats via her work as a skin therapist requiring frequent use of her hands. The culture result showed a growth of gram-negative bacilli and evidence of non-tuberculous mycobacterium. The type of non-tuberculosis mycobacterium was confirmed as Mycobcterium massiliense by NTM polymerase chain reaction (PCR) and hybridization.
After the surgery, the patient received intravenous antibiotic treatment (clarithromycin, 250 mg/day) for 7 days during her hospital stay. A further 4 months of oral antibiotics (clarithromycin, 1,000 mg/day) was prescribed to ensure complete remission. During the 6-month follow up period, there were no recurrences and the patient recovered uneventfully (Fig. 5).
Discussion
CSD is an infectious disease mainly caused by Gram-negative Bartonella henselae. Although an accurate mechanism for transmission between cats and humans is not yet known, according to Mehmet et al. [3], 14 out of 18 CSD patients (77.7%) had a history of cat exposure. Severity and presentation of the disease are related to the immune status of each individual patient. In general, immunocompetent patients who are otherwise healthy tend to present with typical CSD. However, immunocompromised patients tend to have the systemic form of the disease [3-5].
The primary lesion at the site of a scratch or bite, usually by a cat, begins with an erythematous papule. The papule appears 3 to 10 days after injection, and progresses through erythematous, vesicular, and crust status. For immunocompetent patients, the process of CSD is usually self-limited to the lesion and dissipates within 3 weeks, but may last for 1 to 3 years in immunocompromised patients [4-6]. However regional LAP of CSD may occur in certain cases, and where enlarged lymph nodes persist, they should be excised surgically [7-9]. LAP occurs most frequently in the axilla (46%), head and neck (26%), and the groin (17.5%) [5]. Other associated symptoms include fever, fatigue, malaise, hepatosplenomegaly, skin rash, headache, sore throat, nausea, abdominal pain, and anorexia [2].
For CSD to be diagnosed, a physical exam is performed to find the inoculation site on the hands, arm, face, chest, and scalp. The Bartonella species are difficult to culture and as a result culturing is not usually recommended [2]. The most sensitive method of confirming CSD is serologic testing. Immunoglobulin G has significant cross-reactivity between B. henselae. Immunoglobulin G titers less than 1:64 suggest a patient does not currently have Bartonella infection. Titers between 1:64 and 1:256 represent a possible infection; repeat testing should be performed in these patients for 10 to 14 days. Titers greater than 1:256 strongly suggest an active or recent infection [10]. Polymerase chain reaction can also detect Bartonella species; specificity is very high, but the sensitivity is lower than serology [11]. If the serologic test fails to verify the diagnosis of CSD, lymph node excision is performed for histopathologic and immunohistochemical examinations. In our case, we did not suspect CSD at first as the patient had no history of direct cat exposure. Thus, a serologic test was not performed initially; instead pathologic findings of the tissue biopsy and immunohistochemical examinations were performed for the diagnosis of CSD.
The pathological findings depend on the stage of CSD. In the early stages of CSD, lymphoid hyperplasia with arteriolar proliferation may be seen. At the end stage of CSD, a star-shaped conformation with multiple microabscesses may appear and a granuloma may accompany the microabscess formation [12,13]. Organisms may be seen within histiocytes, and in necrotic tissue and thrombosed vessels. Warthin-Starry silver staining has proven valuable in the detection of B. henselae [13]. B. henselae may be observed by staining during the early stage, but B. henselae is rarely detected during the later granulomatous stage of inflammation [12].
The antibiotic regime of CSD depends on disease presentation. Most patients do not need antibiotics because they have self-limited lymphadenopathy after 2−8 weeks. However, antibiotics may be helpful if there are systemic symptoms. Azithromycin and clarithromycin, macrolide antibiotics, are treatment choices for CSD. Other antibiotics that have been prescribed in CSD include ciprofloxacin, trimethoprim/sulfamethoxazole, rifampin, and gentamicin [14].
To the best of our knowledge, in Korea, not many cases of axillary lymphadenopathy of CSD have been reported [15]. In our case, an immunocompetent patient presented with axillary LAP and was confirmed to have CSD through a lymph node biopsy despite the patient having no direct contact with cats.
In conclusion, CSD should be under differential diagnosis when patients have a history of frequent contact with cats and present themselves with primary injection site lesion and or regional lymphadenopathy. CSD is diagnosed by the detection of silver-deposited bacilli upon Warthin-Starry staining or by polymerase chain reaction of microbial deoxyribonucleic acid. Serological and culture examinations can also be used for diagnostic purposes. An accurate pathological diagnosis and appropriate culture examination will lead to accurate and effective treatment in lymphadenitis patients.