Introduction
If blood supplies to specific tissues are occluded or cut off, the tissue eventually undergo coagulative necrosis and tissue injury. Reperfusion is achieved to recover tissue damage, but this process paradoxically can itself induce and exacerbate a number of complications such as acute inflammatory responses, necrosis, apoptosis, and metabolic disorders, an entity termed ischemia-reperfusion injury (IRI) [1].
IRI is a pathologic process in which multiple inflammatory responses may take part. Despite the pathophysiology of IRI remains controversial, several factors including necrosis, tissue hypoxia, apotosis, oxidative injury, and inflammatory response appear to promote the development of IRI [2]. Literature has reported that proinflammatory cytokines are key factors leading to immune responses in IRI, since they lead to the accumulation of inflammatory immune cells and the release of inflammatory molecules, several proteases, and reactive oxygen species (ROS) [3,4]. Therefore, pharmacological agents that can inhibit these key of proinflammatory cytokines may facilitate control of IRI.
Cyclopentylamino carboxymethylthiazolylindole (NecroX) compounds were recently discovered as novel necrosis inhibitors with a powerful antioxidant property due to its mitochondrial ROS and peroxynitrite scavenging ability [5]. NecroX-5 (C25H31N3O3S·2CH4O3S; Fig. 1) is a derivative of the NecroX series of compounds. Significant improvement in the protection of cell and tissue has been showed against various injury factors, such as neomycin, nitroprusside, oxidative stress, and gentamicin [5-7]. Recently, the fundamental role of NecroX-5 in IRI in the heart and liver has been described, however, there has been no research to determine the molecular mechanisms underlying the inhibition of NecroX-5 in IRI [8,9]. The goal of this research was to investigate the therapeutic efficiency and its underlying mechanism of NecroX-5 on the IRI in rat flap model.
Methods
Materials
NecroX-5 was provided by LG Life Sciences (Daejeon, Korea). In addition, we purchased primary antibodies against tumor necrosis factor (TNF)-α (sc-1350; 1:250), interleukin (IL)-1β (sc-7884; 1:250), IL-6 (sc-1265; 1:250) from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Hematoxylin and eosin (H&E) staining, ethanol, hydrochloric acid, and xylene were purchased from Duksan Pure Chemicals (Ansan, Korea).
Animals
All animal experiments were conducted in accordance with the guidelines of the approval of the Institutional Animal Care and Use Committee (approval number: BA1103-079/017-01). Male Sprague-Dawley rat of about 300 g were used in this study. With every other condition homogenized, the animals were kept at 21±2°C, controlled humidity of 50±15%, pathogen-free conditions, and light/dark cycles for 12 hours each. Mice were allowed free access to food and water. Every rat was caged separately to avoid flap injury by cannibalism.
Study model
Animals were randomly into the following two groups (n=10 per group) depending on the experimental treatment: control saline group and NecroX-5 group. For the surgical procedure, rats were anesthetized by an intraperitoneal injection of Zoletil (Virbac, TX, USA) at a dosage of 50 mg/kg and inhalation of isoflurane. Thereafter, the surgical area of skin was shaved with clippers and sterilized with 70% ethanol. The superficial inferior epigastric artery based flap measuring 2 cm in width and 9 cm in length was designed and performed on the 20 rats, each side being 1.5 cm from the midline [10]. Ischemia was induced by placing a micro clamp on the flap’s vascular pedicle for 7 hours. Saline or 30 mg/kg NecroX-5 was administered 30 minutes prior to micro clamp insertion and 30 minutes after clamp removal. Interrupted sutures were applied at the caudal left corner part of the flap. Rats were permitted to recover from anaesthesia during the ischemic period. After 7 hours of ischemia, the flaps underwent 24 hours of reperfusion in order to induce IRI. After all these procedures were completed, tissue specimens were collected from 20 rats (10 rats per group). The flap was sutured back into its original location with a wedged-on cutting needle. All surgical procedures were performed by one researcher (B.H.S.) and no rats died during surgery.
Evaluations
On days 7 after the operation, the flap survivability was evaluated by measuring the necrotic surface area. Flap survival was assessed visually and digital images were taken (8MP 19300 Galaxy SIII; Samsung, Seoul, Korea) for further objective analysis using the Image J program (National Institute of Health, Rockville, MD). Flap necrosis was determined based on skin colour, eschar formation, and lack of capillary refill, and the viable area was expressed as a percentage of the total flap area. After the necrotic area was measured, tissue samples were taken from the flap and embedded in paraffin. Sections of flap tissues were stained with H&E, immunohistochemical stain (for TNF-α, IL-1β, and IL-6) before dewaxing and dehydration. Sections were coded and randomly analyzed by two independent blind observers.
Statistical analysis
All data were presented as the mean±standard deviation (S.D.). Statistical evaluation of the survival rate of skin flaps was performed with SPSS version 21 (IBM corporation, Armonk, NY). The differences among groups were analyzed by one-way ANOVA. Post-hoc comparisons were done using Scheffe’s method. The results were considered significant at a value of P<0.05.
Results
Effect of NecroX-5 on flap survival
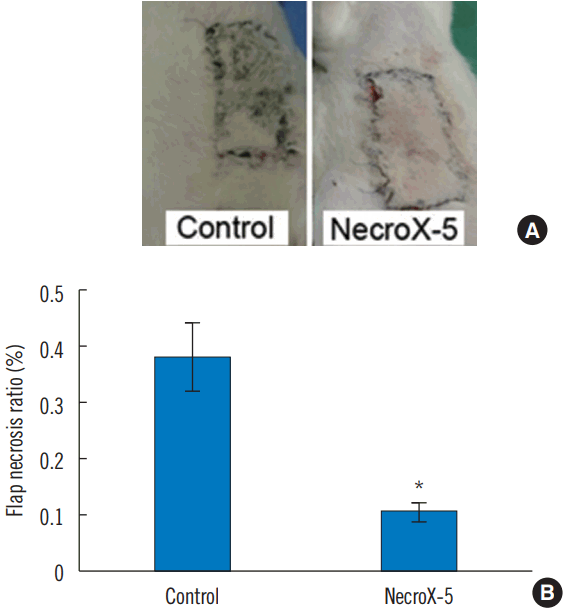
A quantitative assessment of the necrosis area is commonly used for the analysis of flap survival [11]. To discern the efficiency of NecroX-5, we measured the survival of flap tissue on the seventh day after surgery. The average necrotic area in the NecroX-5 group was significantly lower than in the control group (NecroX-5 group: 6±0.019%; control group: 38± 0.11%; P<0.05; Fig. 2).
Effect of NecroX-5 on the levels of proinflammatory cytokines
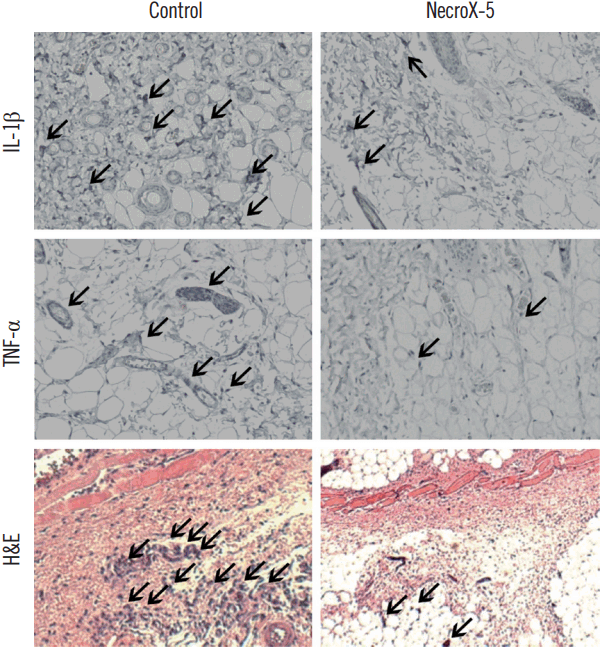
Abundant proinflammatory cytokines were found in IRI [12]. To investigate the mechanisms by which NecroX-5 cause an enhancement of skin flap survival, we detected the production of proinflammatory cytokines, including IL-6, TNF-α, and IL-1β by immunohistochemistry staining. The expression of IL-1β and TNF-α were markedly lower in NecroX-5 groups than in control groups (Fig. 3). No difference of IL-6 expressions among control and NecroX-5 treatment groups were observed (Fig. 3). In addition, NecroX-5 treatment resulted in an apparent reduction in the number of inflammatory immune cells (Fig. 3).
Discussion
Use of skin flap has become widespread in plastic and reconstructive surgery field to repair various surgical defects [13]. Local skin flaps have a better skin color match, thickness, and texture, but the distal portion of skin flap necrosis is a common complication due to the lack of specific vessels and their blood supply [14,15]. In order to avoid flap necrosis, the development of vascular anatomy and the development of surgical procedures such as flap designs have been achieved. In addition, various in vitro studies have been conducted.
Previous studies have shown that several factors, such as inflammatory reactions, inadequate angiogenesis, and oxidative stress appear to cause the development of flap necrosis [16,17]. These findings prompted the investigation of promising pro-angiogenesis, anti-inflammatory, and oxidative stress reducing agent to improve flap survival [18,19]. Smooth muscle relaxants, alpha receptor blockers, or axon blockers has been applied to enhance circulation in skin flaps through the increase of erythrocytes flexibility and blood viscosity. Moreover, antioxidants and anti-inflammatory agents have been applied to suppress the cellular effects of ischemia [20].
Recently, anti-necrotic effects of NecroX-5 on IRI have been evaluated in the murine heart and canine liver IRI models [8]. NecroX-5 strongly prevented myocardial damage in ischemic heart disorders by preventing mitochondrial dysfunction [8]. In addition, it is reported that NecroX-5 was able to enhance the viability of skin flaps in murine model [21]. Intraperitoneal injection of NecroX-5 significantly increased the survival of dorsal skin flap [21]. In the present study, we also investigated the effect of NecroX-5 on skin flap survival. Ischemia was induced by placing a micro clamp on the flap’s vascular pedicle for 7 hours and saline or 30 mg/kg NecroX-5 was administered 30 minutes prior to micro clamp insertion and 30 minutes after clamp removal. Consistent with previous study, we showed that NecroX-5 significantly decreased the necrotic area of flap in IRI rat model. However, further studies need to investigate whether the therapeutic effect of NecroX-5 on skin flap survival was established 30 minutes before or 30 minutes after the vessel clamp.
Inflammation is a plausible pathway in the development of IRI, because it plays an important role in cellular necrosis and apoptosis [22]. During reperfusion, inflammatory immune cells can enter the zone of ischemic injury and become activated. They produce multiple inflammatory cytokines like IL-1, TNF-α, and IL-6, which is tightly correlated to the tissues with IRI [12]. A study by Gurevitch reported that anti-IL-6 or anti-TNF-α therapy was able to induce the myocardial recovery after ischemic and reperfusion [23]. Also, there are studies that Necrox-5 suppressed the levels of pro-inflammatory cytokines like IL-6 and TNF-α through the inhibition of Akt/nuclear factor-κB signaling pathway in a rat model of hypoxia/reoxygenation [8,24]. Therefore, we hypothesized that NecroX-5 exerts anti-necrotic effect by modulating the production of proinflammatory cytokines. As expected, NecroX-5 suppressed the secretion of TNF-α and IL-1β in IRI model. This result indicated that NecroX-5 increased the flap survival after IRI via the inhibition of proinflamatory cytokines production. However, further studies are required to investigate the exact signaling mechanism mediating the anti-necrosis induced by NecroX-5.