Surgical Approach to Necrotizing Fasciitis in the Buccal Fat Pad
Article information
Abstract
Cervicofacial necrotizing fasciitis (CNF) of the face is a rare and potentially life-threatening bacterial infection that requires immediate intervention. CNF involving the buccal fat pad particularly demands surgical drainage, with attention to the surrounding anatomical structures to prevent vascular or nerve damage. In this study, we reviewed the anatomy of buccal fat pads to suggest appropriate surgical approaches. A retrospective chart review was conducted on seven patients with CNF who had a buccal fat pad abscess requiring surgical incision and drainage between January 2022 and August 2023. Abscesses within the central buccal fat pad and its pterygoid extensions were drained via intraoral incisions. Abscesses in the temporal extension were addressed by the Dingman approach. All patients underwent our surgical drainage regimen combined with proper intravenous antibiotics, leading to successful treatment of CNF without significant functional sequelae, with an average stay of 18.71 days. The buccal fat pad, which corresponds to the deep space of the face, is surrounded by vital structures such as the facial artery, vein, nerve, and parotid duct. When treating abscesses in the buccal fat pad, it is important to understand the relationship of the fat pad to other vital structures for optimal outcomes.
Introduction
Cervicofacial necrotizing fasciitis (CNF) is a rare condition in which a bacterial infection spreads along the facial fascia. If left untreated, this condition can lead to significant mortality; therefore, timely and appropriate treatment is essential. The three pillars of treatment for necrotizing fasciitis (NF) are drainage of pus, eradication of the infectious origin, and appropriate administration of antibiotics [1]. For CNF occurring in the buccal fat pad, which corresponds to the deep space of the face, surgical drainage remains pivotal. However, the face has a layered structure, with major vessels and facial nerves traversing complex pathways, and the aesthetic outcome post-treatment is of significant importance. Additionally, the buccal fat pad is a three-dimensional structure with complex anatomy. Therefore, creation of an incision to reach an abscess pocket in the deep space of the face, as one might for other areas, can be challenging. In this study, we review the anatomy of the buccal fat pad and methods of surgically draining abscesses in this location. We also compile a case series on the treatment.
Idea
Patients
From January of 2022 to August of 2023, we conducted a study of patients with CNF at three hospitals. A retrospective chart review was carried out to collect cases of patients hospitalized due to CNF, specifically those who had a buccal fat pad abscess requiring surgical incision and drainage. Additionally, we collected data on the patients’ medical history, Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) scores, and interpretations of computed tomography or magnetic resonance imaging (MRI). This report was approved by the Institutional Review Board of the Catholic University of Korea(IRB No. XC23RADE0103). The patients provided written informed consent for the publication and the use of their images.
Anatomy of the buccal fat pad
The buccal fat pad is a structure located in the buccal space. Anteriorly, it is bordered by the facial expression muscles; posteriorly by the masseter muscle, mandible, and lateral and medial pterygoid muscles; medially by the buccinator muscle; and laterally by the superficial layer of the deep cervical fascia. The buccal fat pad was classically described by Stuzin et al. [2] as consisting of a main body and four extensions [2]. The four extensions are the superficial part known as the buccal extension, the deep and posterior part called the pterygoid extension, and the superior parts termed the superficial and deep temporal extensions.
Using the parotid duct as a reference, the buccal extension is located inferior to the duct, while the main body is superior to it. The opening of the parotid duct is opposite the second maxillary molar. The main body wraps around the posterior and medial aspects of the maxilla as it extends posteriorly. In this region, it envelops the internal maxillary artery and the maxillary division of the trigeminal nerve. The deep part of the temporal extension is located deeper than the zygomatic arch and is more superficial than the temporal muscle and its tendon. It passes superiorly behind the medial aspect of the zygoma body and the lateral orbital wall [2].
Zhang et al. [3] describe the buccal fat pad using a different model, identifying anatomically distinct anterior, intermediate, and posterior lobes. The anterior lobe is triangular in shape and situated below the zygoma, deeper than the mimetic muscles but anterior to the buccinator and maxilla. This lobe envelops the infraorbital vessel and nerve and is overlaid by branches of the facial nerve; the parotid duct passes posterior to the anterior lobe. The intermediate lobe is located on the lateral maxilla, positioned between the anterior and posterior lobes. The posterior lobe, situated in the masticatory space, possesses four extensions: the buccal process, which is the most superficial and passes below the parotid duct; the pterygopalatine process, which encases the pterygopalatine fossa and vessel; the pterygoid process located in the pterygoid space; and the temporal process. Following Stuzin et al. [2], the temporal extension is divided into superficial and deep parts. The portion connected to the main body of the buccal fat pad is the deep part, while the superficial part is encapsulated by the deep temporal fascia as a distinct structure. However, Zhang et al. [3] determined that the anterior end of the superficial part is connected to the deep part at the anterior rim of the temporalis muscle.
Surgical technique
There are two methods that can be used to safely access an abscess located in the buccal fat pad. First, an intraoral incision is used to drain the abscess from the main body as well as the buccal and pterygoid extensions. During this process, the incision is made either 1 cm behind [2] or 1 cm below [4] the parotid duct (Stensen’s duct) opening to avoid injury to the parotid duct, facial artery and vein, and facial nerve (Fig. 1). Second, the Dingman approach is employed to drain the temporal extension, which involves an incision lateral to the eyebrow [5]. When traversing from the temporal extension to the main body, tunneling is carried out alongside the lateral orbital wall using a Dingman elevator (Fig. 2).

Illustration of an intraoral incision. The intraoral incision is made either 1 cm behind or 1 cm below the parotid duct (Stensen’s duct) opening to avoid injury to the parotid duct, facial artery and vein, and facial nerve.
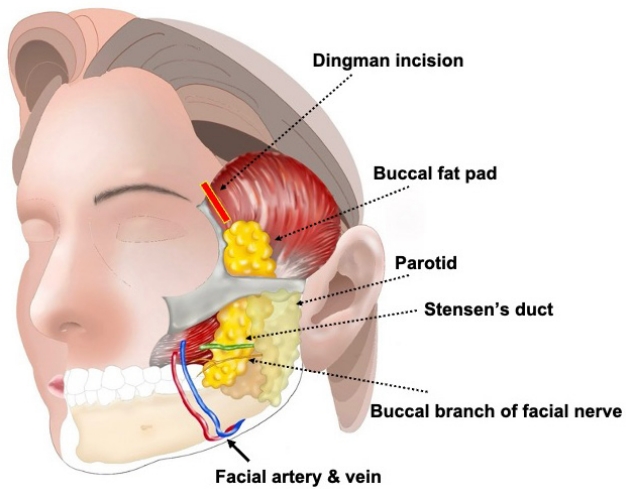
Illustration of a lateral eyebrow incision (Dingman approach). The Dingman approach is employed to drain the temporal extension, which involves an incision lateral to the eyebrow. The buccal branch of the facial nerve, along with the artery and vein, passes inferiorly to Stensen,s duct, which lies on the buccal fat.
The appropriate approach would be based on the location of the abscess in the buccal fat pad. If an abscess extends across the entire buccal fat pad, connecting the Dingman approach to the intraoral incision aids in draining it effectively.
Results
A total of seven patients were included in our retrospective chart review. The demographics of the patients are presented in Table 1. The average age was 56.14 years (range, 33–75 years), and the proportion of males was 57.14%. The average LRINEC score and length of hospital stay were 5.86 (range, 1–13) and 18.71 days (range, 7–52 days), respectively. Of the patients, three had underlying diseases of hypertension, diabetes, and kidney disease, while the other four had no underlying conditions. The etiologies of the patients’ conditions were as follows: odontogenic in four cases, trauma in two cases, and unknown in one case.
All patients had abscesses in the buccal space, and additional abscesses were found in the perioral, orbital floor, parapharyngeal, retropharyngeal, carotid, and submandibular spaces. Abscesses occurring in the buccal fat pad, excluding those with temporal extension, were all surgically drained using an intraoral approach. Abscesses with temporal extension were addressed using the Dingman approach. All patients were discharged in a state of improved infection, with no cases of recurrence, significant functional sequelae, scar contracture or fascial asymmetry for an average of 5.42 months (range, 3–9 months) of follow-up.
Case presentation
Case 1
A 50-year-old female without any underlying history presented to the emergency department with a chief complaint of right facial swelling (Fig. 3). She had received a dental implant at the right upper second molar 2 weeks before showing symptoms. The implant was removed 1 day before she arrived at the emergency department.

Initial clinical presentation of case 1. Right-side facial swelling involving the periorbital area accompanied by pain, tenderness, erythema, and heating sensation. (A) Front view. (B) Right lateral view.
At the initial screening, prominent right-side facial swelling was observed, extending to the periorbital area. Her white blood cell count was 11,300/μL and C-reactive protein was 15.87 mg/dL. Her LRINEC score was 7 points. An MRI scan revealed diffuse fluid collection with internal air density in the right masticator and buccal spaces, extending temporally (Fig. 4). This suggested NF. There was also a subperiosteal abscess on the right orbital floor, and the right maxillary sinus was filled with pus. Therefore, empirical intravenous antibiotics were administered, and surgical drainage was planned.

Initial face magnetic resonance imaging of case 1. A probable subperiosteal abscess in the right orbital floor was shown in the T2-weighted coronal image. An irregular rim enhancing lesion with an internal air-fluid level in the right buccal and masticator space suggesting necrotizing fasciitis was evident in T2-weighted coronal and axial images. (A) T2-weighted coronal image. (B) T2-weighted axial image.
Under general anesthesia, a Caldwell-Luc operation was performed along with incision and drainage via the Dingman approach. Initially, an incision was placed in the right upper gingivobuccal sulcus, extending from the canine to the last molar. An anterior antrostomy was then performed using an electronic burr. Following this, necrotic and infected tissue, including the fascia, was excised, and the area was irrigated with saline. Next, a right subbrow incision was created to drain the abscess from the deep temporal extension. A tunnel was created with a Dingman elevator extending to the last molar to expose the main body of the buccal fat. The abscess on the orbital floor was drained through a transconjunctival incision. A silastic drain was inserted at each incision site, with the exception of the transconjunctival incision (Fig. 5).

Postoperative clinical photo of case 1. Drainage of pus was performed through incisions in the right subbrow and intraoral areas. A silastic tube was inserted for continuous drainage in the subbrow through intraoral areas. This photo shows mitigation of swelling 7 days after the operation.
Sequential debridement and wound irrigation were performed daily. Blood cultures showed no bacterial growth, while the cultured abscess revealed mixed growth of Streptococcus anginosus, Staphylococcus epidermidis, and Neisseria subflava. A selective antibiotics regimen was initiated with intravenous amoxicillin/clavulanate (1.2 g every 12 hours) and oral doxycycline (100 mg every 12 hours). The patient was discharged from the hospital 18 days after admission with improved signs of infection.
Case 2
A 75-year-old male patient with a medical history of hypertension, end-stage renal disease requiring regular dialysis, and coronary artery disease treated with a stent insertion presented to the emergency department complaining of left facial swelling (Fig. 6). One week before the onset of this symptom, he had undergone periodontal curettage at a local dental clinic, and a tooth extraction had been scheduled.
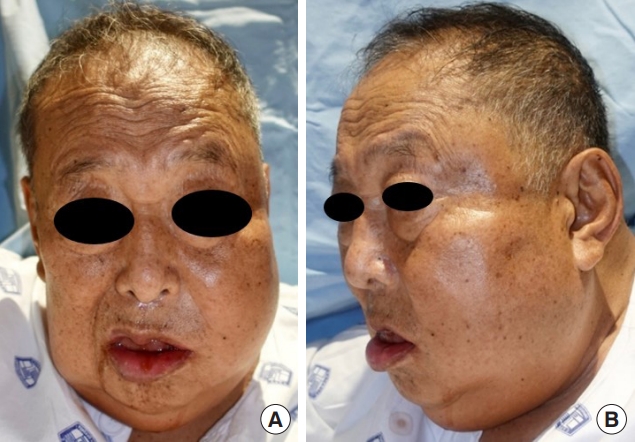
Initial clinical presentation of case 2. Swelling in both cheeks, more prominently in the left cheek, was observed along with pain, tenderness, erythema, and heat sensation. Pus drainage in the left lower periodontal area was noted. (A) Front view. (B) Left lateral view.
Upon initial physical examination, prominent swelling was noted in the mandibular area, especially on the left side. Pus was observed draining around the roots of the left upper second and third molar teeth. The total white blood cell count and C-reactive protein level were elevated, measuring 18,600/μL and greater than 34.1 mg/dL, respectively. His LRINEC score was 13 points. An MRI scan revealed an abscess pocket in the left masticator, submandibular, and parapharyngeal spaces, suggesting odontogenic origin with deep neck infection (Fig. 7). Intravenous antibiotic treatment was promptly initiated, followed by hospital admission. We planned to surgically drain the body, buccal, and pterygoid extensions of the buccal fat and the masticator space.
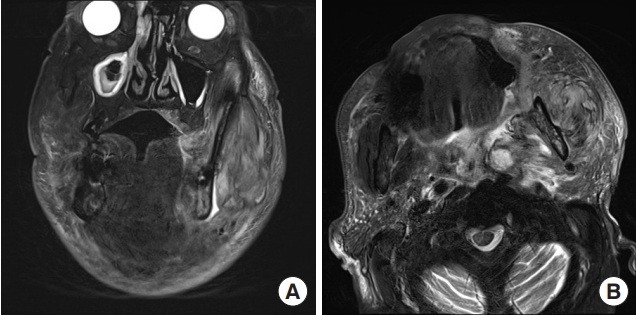
Initial face magnetic resonance imaging of case 2. Both T2 axial and coronal images showed heterogeneously enhancing lesions involving the left masticator space, parapharyngeal space, retropharyngeal space, carotid space, and submandibular space. Internal air-fluid density suggested abscess formation. (A) T2-weighted coronal image. (B) T2-weighted axial image.
Surgical drainage was performed under general anesthesia. The teeth responsible for the infection were extracted. The abscess in the main body, buccal, and pterygoid extension of the buccal space was drained via an intraoral incision along the lower border of the zygomatic arch. The submandibular abscess was drained from the lower border of the zygomatic arch through to the lower border of the mandibular body. The parapharyngeal, retropharyngeal, and carotid space abscess was drained by lateral neck incision (Fig. 8). A silastic drain was inserted into each tunnel, and daily continuous irrigation and drainage were carried out.

Postoperative clinical photo of case 2. Extensive pus drainage was performed through incisions in the left cheek, posterior auricular area (A), and buccal mucosa (B). Silastic tubes were inserted in each of the incisions, and one of them penetrated two cheek incisions.
Tissue culture obtained during the surgery revealed growth of Klebsiella pneumoniae ssp. Consequently, the antibiotic regimen was adjusted to intravenous ceftriaxone (1 g every 24 hours) and clindamycin (600 mg every 8 hours). The patient’s condition steadily improved; he was discharged on the 52nd day.
Discussion
NF is an infection that progressively deteriorates, spreading along the fascia, including the subcutaneous tissue and skin. During this process, ischemic necrosis of the tissue occurs due to severe inflammation accompanied by a reduction in blood flow, which is caused by local vascular thrombi in the soft tissue [6]. The clinical presentation includes pain, swelling, and erythema. NF spreads along the fascia, which is a poor vascular plane, allowing faster spread than in other soft tissue infections. NF in the cervical region is relatively uncommon, comprising up to 10% of total cases, due to abundant blood circulation [7]. Nevertheless, if CNF is neglected without appropriate treatment, it can lead to unsightly facial disfigurement, facial-disabling functional deficits, and even death [8]. Therefore, timely and appropriate treatment is essential.
In a study using national audit data from the United Kingdom, CNF occurred in about 5.3% of NF patients, most of which were of dental infection origin, mainly from the maxillary or mandibular molars [9]. As a result, abscesses frequently occur in the buccal and masticator spaces. Additionally, CNF can develop in the parotid space enclosed by the deep cervical fascia, the peritonsillar space demarcated anteriorly and posteriorly by the tonsillar pillars, medially by the palatine tonsil, and laterally by the superior pharyngeal constrictor, as well as in the periorbital area [10].
In 2004, Wong et al. [11] introduced the LRINEC score as a system based on routine laboratory tests for evaluating soft tissue infections that can identify early NF. Based on clinical presentation or physical examination, a score of 6 or higher is considered intermediate risk or above, and urgent MRI is recommended. If the patient is subsequently diagnosed with NF, emergent surgical drainage is advised. According to a meta-analysis conducted by Kim et al. [12], the LRINEC score is also useful for predicting NF in cases involving the head and neck. However, even if the LRINEC score is lower than 6 points, NF may be present, necessitating surgical debridement if clinically suspected [13]. In the present study, patients with low LRINEC scores (patient numbers 3 and 6) were assessed and subsequently underwent surgical incision and drainage due to clear signs of abscess, including erythema, swelling, fluctuation, and purulent discharge.
When draining an abscess in the buccal fat pad, it is essential to be aware of its relationships with vital structures. Cotofana et al. [14] described the relationships between the buccal fat pad and superficial structures. The buccal fat pad is located deeper than the parotid masseteric fascia, often referred to as the fifth layer of the face. The parotid duct, facial vein, and the buccal branch of the facial nerve are all superficial to the posterior laminar of the parotid masseteric fascia. Hence, they run more superficially than the buccal fat pad. Therefore, to access the main body and buccal extension of the buccal fat, the incision is either 1 cm behind [2] or 1 cm below [4] the parotid duct opening or at the upper buccal sulcus located at the last molar tuberosity [15]. To access the temporal extension, an incision next to the lateral eyebrow allows a direct approach to the deep part using the Dingman approach. When combined with an intraoral incision, the entire buccal space can be drained. At this point, caution is needed to ensure that the Dingman elevator does not enter the posterior region of the maxilla traversed by the internal maxillary artery and the maxillary division of the trigeminal nerve.
To treat CNF abscesses in the buccal fat pad, which is one of the facial deep space structures, a comprehensive understanding of the anatomy and familiarity with the surrounding structures such as the facial artery, vein, nerve, and parotid duct is crucial. By using intraoral incisions and the Dingman approach, it should be feasible to securely perform surgical drainage of an abscess within the buccal fat pad and achieve an effective treatment outcome.
Notes
No potential conflict of interest relevant to this article was reported.

