Reconstruction and Management Strategies for Pelvic Ablative Surgery
Article information
Abstract
Background
Ablative oncologic procedures for colorectal or gynecologic malignancies can result in large skin or tissue volume defects. Although direct closure may be possible, such attempts can lead to postoperative complications such as wound breakdown, organ prolapse, chronic seroma, or infection. Various procedures, from flap surgery to local wound care, can be useful additions to improve patient outcomes.
Methods
This study retrospectively reviews cases of patients with multiple comorbidities who had undergone concomitant interventions after pelvic ablative surgery. Various interventions after pelvic ablative surgery, from reconstructing the defect to managing postoperative complications, are described.
Results
Careful planning and selection of the reconstruction method can significantly improve patient outcomes. The authors suggest using gluteal flaps for most reconstructive demands.
Conclusion
This case series emphasizes the utility of using various flaps, especially the gluteal flap, in reconstructing oncologic defects in the pelvic and perineal regions. The insights gained from this study will hopefully be of assistance to future research and clinical practice, ultimately improving patient outcomes.
Introduction
There are increasingly diverse surgical indications for locally advanced colorectal/gynecologic cancer [1]. Reconstruction of the pelvic and perineal areas following oncologic surgeries, such as those for locally advanced colorectal or gynecological malignancies, presents significant challenges. Abdominoperineal resection (APR), pelvic exenteration (PE), and debulking surgery are commonly used to treat advanced or recurrent cancer in the pelvic region [2]. These procedures involve the removal of cancerous tissue and are considered radical due to the extent of the resection. The procedures demonstrate a high rate of postoperative complications, especially perineal wound complications [3].
Specifically, PE has emerged as a widely accepted therapeutic approach for advanced pelvic malignancies that were previously considered unresectable [4]. However, the radical removal of the skin, soft tissue, and bony structures in the pelvis creates an excessive intra-abdominal void and perineal defects, leading to complications such as pelvic fluid collection, bowel obstruction, fistulae, and perineal wound breakdown [5]. The so-called “empty pelvis syndrome” poses an ongoing challenge for exenteration surgeons, and the benefits of improved survival through clear surgical margins must be weighed against the morbidity associated with acute, chronic, and long-term pelvic complications [6]. To overcome this drawback, oncologic surgeons can benefit from collaborating with reconstructive teams and proceed confidently with pelvic ablation.
Reconstruction in patients with locally advanced colorectal cancer presents unique challenges to reconstructive surgeons because most patients share multiple risk factors, such as (1) old age, (2) a history of open abdominal surgery, (3) a history of pelvic irradiation, (4) comorbid medical condition, (5) decreased general performance status, and (6) other overlapping malignancies. Moreover, compared to reconstructions in the same area requiring skin-only coverage, pelvis and perineum reconstruction after pelvic ablative surgery requires additional preoperative considerations. This is because pelvic ablative surgery involves significant dead space volume, the proximity of the intra-abdominal organs, increased susceptibility to bacterial infection, and extended periods of direct wound pressure [7]. Even in cases where tissue transfer is unnecessary, wound complications may pose serious problems after pelvic ablative surgery due to the multiple comorbidities of the patient. These problems often require surgical or nonsurgical interventions from reconstructive surgeons.
This case series aims to provide valuable insights into the surgical and nonsurgical management of patients undergoing oncologic resection and reconstruction in the pelvic and perineal areas for various etiologies.
Methods
This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (IRB No. 2023-2685-0001). The study was performed in accordance with the principles of the Declaration of Helsinki. The patients provided written consent for the use of their photographs. The retrospective study enrolled patients who received joint surgery or treatment by the colorectal and plastic surgery teams. Among the patients who required surgery or wound management consultations between January 2020 and December 2022, patients with colorectal malignancy were selected. An electronic medical chart review was undertaken to gather patient data, including demographics, related systemic diseases, oncologic diagnoses, tumor staging, reconstruction methods, and adjunct treatments.
Results
A total of nine patients who received surgical and nonsurgical treatment were eligible for inclusion in this retrospective cohort study. The demographic and operative information is summarized in Table 1. Among the nine patients included in this series, cases 1 and 2 were reconstructed with muscle-only flaps and separate local flaps for skin coverage. Cases 3 to 6 were reconstructed with gluteus-based flaps. Case 7 was reconstructed with an inner thigh flap. One patient (case 1) had cancer recurrence after primary reconstruction. In most cases, adjuvant therapy, either chemotherapy or radiotherapy, was applied after reconstruction (cases 2, 3, 4, 5, 6). Flap necrosis developed in one case (case 1). Detailed descriptions of representative cases are provided below.
Reconstruction after PE (cases 1 through 4)
Case 2
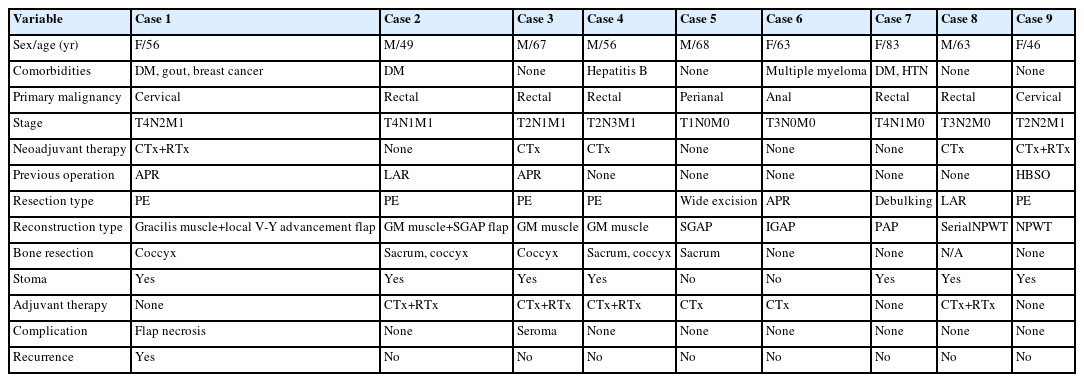
A 49-year-old male patient with a surgical history of T-loop colostomy and postoperative chemotherapy for rectal cancer presented with multiple metastases involving the rectum, liver, prostate, and lymph nodes. PE including radical cystectomy and ureterocutaneostomy was performed. Finally, the peritoneal defect after sacrectomy was covered with biologic mesh. To fill the volume defect after sacrectomy, the void was padded by bilateral gluteus maximus interdigitation flaps using the vest-over-pants method. For skin coverage, two separate bilateral gluteal maximus fasciocutaneous flaps based on perforators from the left and right superior gluteal artery were elevated (Fig. 1A). These faciocutaneous flaps were advanced toward each other, and the redundant skin of the right side was de-epithelialized and buried to provide additional padding (Fig. 1B). This interdigitation enabled robust padding and volume restoration over the sacrectomy defect. The wound healed without any major complications (Fig. 1C).
Case 3
A 67-year-old male patient with a history of rectal cancer presented with recurring rectal cancer. He remained cancer-free for 4 years after conversion APR and postoperative radiotherapy. Enhanced computed tomography (CT) of the abdomen and pelvis revealed a recurrent tumor in the presacral space, which was extending into the prostate gland and posterior wall of the urinary bladder. Neoadjuvant chemotherapy was provided before PE. After pelvic exenteration, biologic mesh was applied. Bilateral gluteal maximus muscle flaps were elevated over the biologic mesh (Fig. 2A), advanced toward each other, and padded the defect at the coccygeal area (Fig. 2B). The patient remains disease-free at the 2-year follow-up.
Case 4
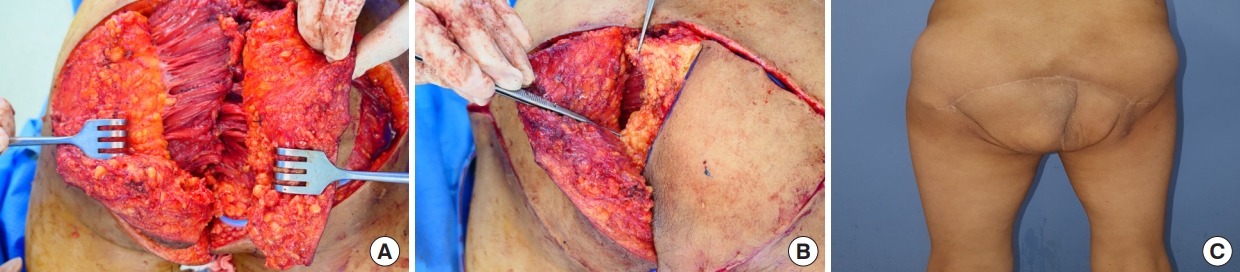
A 56-year-old male patient with a history of rectal cancer presented with recurring rectal cancer. He was treated with neoadjuvant concurrent chemoradiation therapy, followed by robot-assisted APR and adjuvant chemotherapy, and remained cancer-free for 3 years. Local recurrence involving the left mesocolon was noted in an enhanced CT of the abdomen and pelvis, and PE was performed by a general surgeon. A biological mesh was fixed to the sacral area after sacrectomy, the defect measuring 15×20 cm. Bilateral gluteus maximus muscle flaps were elevated and approximated to pad the bone defect (Fig. 3A). The wound healed without any major complications. On long-term follow-up of 3 years, the patient did not experience difficulties in sitting or squatting (Fig. 3B).
Reconstruction after wide skin excision/APR/debulking surgery (cases 5 through 7)
Case 6
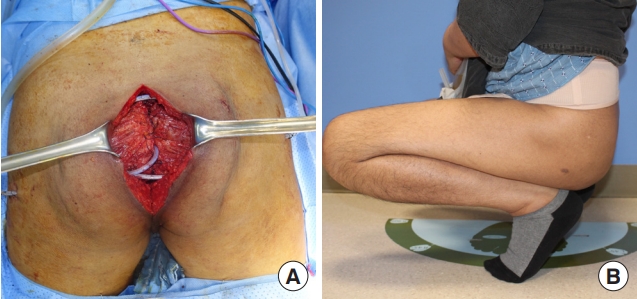
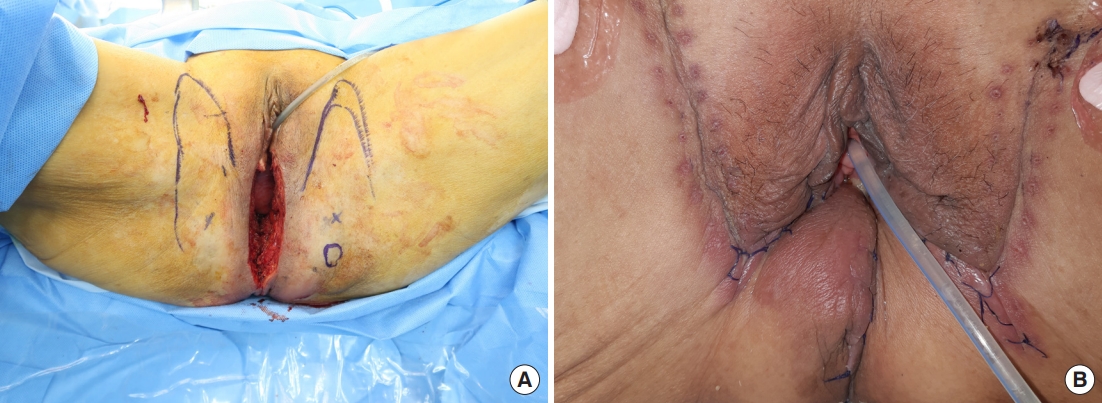
A 63-year-old female patient presented with a 1-year history of a perianal fistula diagnosed with mucinous adenocarcinoma. She had an underlying history of multiple myeloma treated with auto-peripheral blood stem cell transplantation. Imaging showed anal cancer with co-existing inflammation invading the left levator ani and external sphincter muscles. Robot-assisted APR was performed; the defect was approximately 7×10 cm in size (Fig. 4A). A perforator flap based on the perforators from the inferior gluteal artery was elevated from the left side (Fig. 4B), and the flap was advanced to the medial side to fill the defect.
Case 7
An 83-year-old female patient presented with an incidental colonoscopy finding of rectal cancer. The initial magnetic resonance imaging and CT revealed extramural vascular invasion with possible invasion of the vaginal, left internal, and external sphincter. The patient was referred after robot-assisted APR and wide excision of the posterior wall of the vagina, with an estimated defect size of 8×10 cm (Fig. 5A). Bilateral pudendal artery perforator flaps were elevated and rotated into the pelvic defect to resurface the posterior surface of the vagina. The wound healed without major complications at the 4-month follow-up (Fig. 5B).
Management of post-resection wound complications (cases 8 and 9)
Case 8
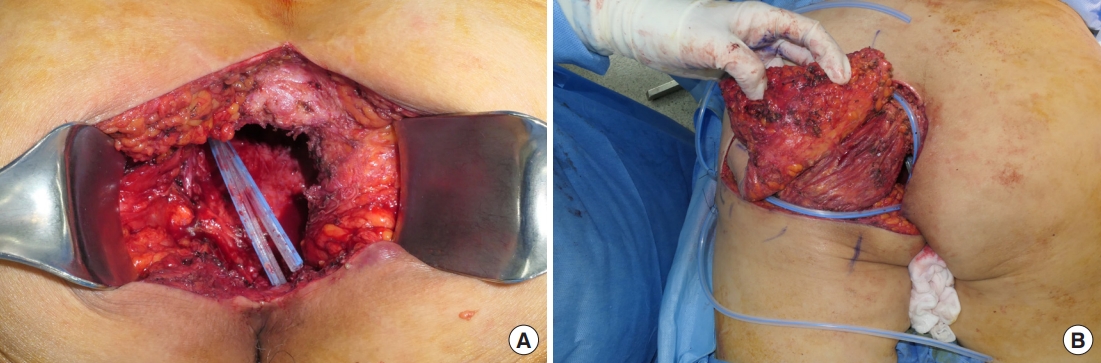
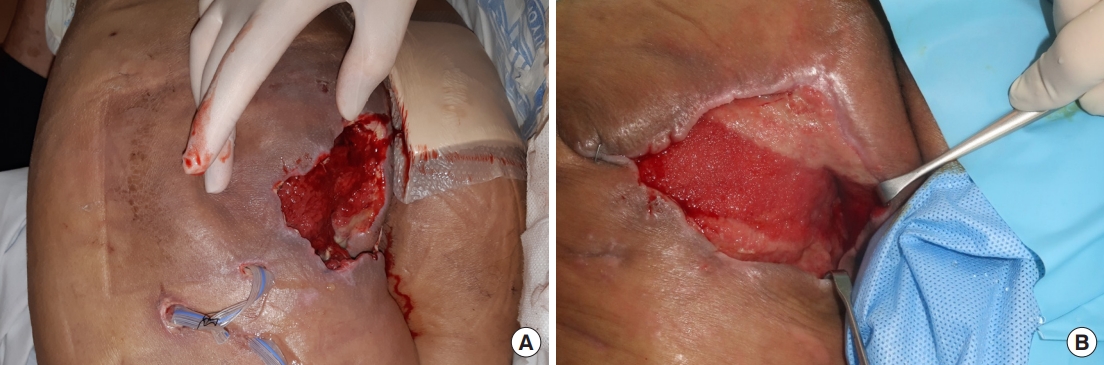
A 60-year-old patient with a history of rectal cancer, treated with robot-assisted low anterior resection followed by adjuvant and palliative chemotherapy, presented with a 10-day history of a perineal abscess. Incision and drainage were performed under general anesthesia (Fig. 6A), and tissue culture showed the growth of extended-spectrum beta-lactamase negative Escherichia coli and Enterococcus faecalis. Serial irrigation was performed, and conservative management with serial negative pressure wound therapy (NPWT) was planned, in consideration of the patient’s poor general condition. The fistula was closed after 5 months (Fig. 6B), and the remnant skin defect was planned to heal with secondary intention.
Case 9
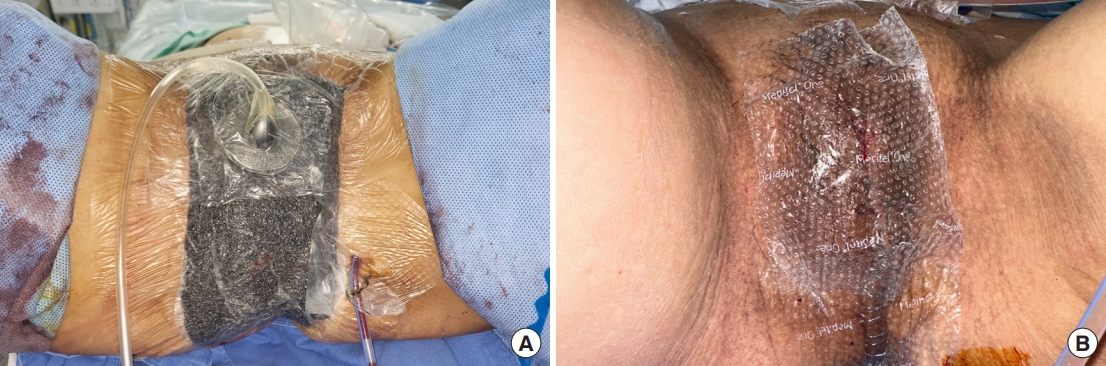
A 46-year-old patient with recurrent rectal cancer from previous cervical cancer. The patient received open APR, total vaginectomy, and radical cystectomy with ileal conduit ureterostomy. Since bone resection was not undertaken, and skin quality was adequate despite previous chemoradiation, an attempt at direct closure was decided. Incisional NPWT (continuous mode, 100 mmHg) was applied to the closed wound to minimize the complication rate (Fig. 7A). NPWT was re-applied for two cycles until the patient gained full ambulatory function. The patient showed a faster healing rate and reduced fluid drainage (Fig. 7B).
Discussion
This retrospective case series aimed to provide valuable insights into the management of patients undergoing oncologic reconstruction in the pelvic and perineal regions. Despite advances in surgical techniques and preoperative planning, reconstruction in this region remains a complex and challenging task [5]. Therefore, investigation of challenges and outcomes associated with oncologic reconstruction in this area is required [3,6].
A reconstructive team is crucial for the successful resection of tumors because the absence of a reconstructive team may hinder the oncologic surgeon’s decision in performing a wide enough resection regardless of the size of the resulting defect. Furthermore, when the resection involves a significant amount of perineal skin, soft tissue, and pelvic floor musculature, not only a wide skin defect but also a large volume defect is created. This large void with thin tissue coverage produces localized weakness in wound tension and leads to long-term complications such as repeated wound dehiscence, minor wound breakdown, chronic seroma, and postoperative ileus. Such constellation of symptoms is termed “empty pelvis syndrome” [8]. Flap reconstruction is highly recommended to prevent the empty pelvis syndrome. Rather than attempting direct closure under tension, providing enough bulk and area of soft tissue and skin by using an appropriate flap to the pelvic area is highly effective in preventing wound breakdown, supporting the intra-abdominal organs, and filling the dead space in the perineal area. A systematic review and meta-analysis by Devulapalli et al. [9] concluded that compared to direct closure, adding reconstruction with a tissue flap helps reduce both major and minor complications. Zeiderman et al. [10] performed an observational study investigating the National Inpatient Sample for 16 years. The study demonstrated a clear trend toward increasing flap reconstruction with pelvic ablative surgery, and concomitant decrease in length of stay and complications.
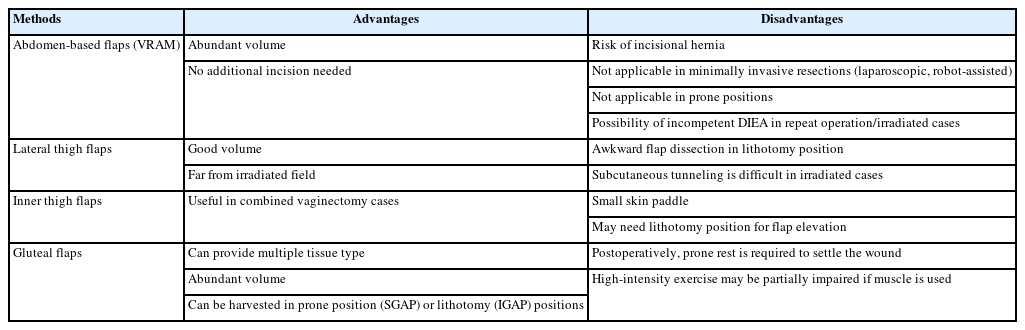
From cases 1 to 7, we wanted to highlight the utility of the gluteal flap over abdomen-based flaps for pelvic reconstruction. Existing literature suggests the vertical rectus abdominis myocutaneous (VRAM) flap as the preferred choice when reconstructing a large volume defect of the perineal area [11,12]. However, based on our experience, we suggest using gluteal flaps as the first-line choice for reconstruction in pelvic ablative surgery on patients with previous surgical history and medical comorbidities. Our inclination toward gluteal flaps also conforms with more recent studies [13-15]. For example, a study by Stein et al. [16] performed a multi-institutional survey on colorectal and plastic surgeons. On analysis, a clear trend toward gluteus-based flap reconstruction after pelvic ablative surgery was observed. Notably, this trend was more prominent in high-volume centers. The authors suggested that this large trend shift from VRAM to gluteal flaps is due to the advancement of minimally invasive resection techniques using laparoscopy or robotic systems, which do not incorporate the midline laparotomy incision. As demonstrated in cases 2 and 3, where APR was performed with a robotic system, VRAM reconstruction is inadequate because performing a separate laparotomy solely for intra-abdominal transfer of the flap is an unrealistic surgical decision [16]. Meanwhile, the gluteal flap is highly suitable for reconstructing large and voluminous pelvic defects; gluteal flaps provide abundant volume and numerous types of tissue, are usually distant from the field of radiation, and are based on a rich and robust vascular supply far from the operative field. Gluteal flaps can be harvested in both prone and supine positions, based on either inferior or superior gluteal artery systems [14,17]. The freedom gluteal flaps provide in positioning the patient for harvesting the flap is a great advantage when sacrococcygeal bone resection is performed, and the patient is presented to the reconstructive surgeon in a prone position. Gluteal flaps provide diverse tissue types that satisfy the volume and surface needs of reconstruction [15]. A volume defect is best restored with a muscle flap, whereas a surface defect is best restored with perforator-based fasciocutaneous flaps with a defatting procedure to conform to the perineal skin curvature.
One limitation of our case series is the absence of reconstruction cases with VRAM and anterolateral thigh (ALT) flaps. This is due to the unique past surgical history of our case series, which mostly comprises of patients with multiple recurrences and radiation history after primary treatment. In reconstruction after primary resection of colorectal malignancies, the use of abdomen flaps, specifically the VRAM flap for reconstructive procedures, has been extensively documented [11,12,18]. The VRAM may be a superb choice in reconstruction after primary cancer in non-obese patient groups. However, the use of VRAM flaps presents challenges in patients who have undergone radiotherapy or multiple laparotomies, as extensive abdominal adhesions can make it difficult to safely isolate the deep inferior epigastric pedicle. Furthermore, previous surgeries may have already severed the deep inferior epigastric artery, rendering the VRAM flap impossible to utilize. In highly obese patients, incisional hernia is also a potential problem. Meanwhile, thigh-based flaps, mainly the ALT flap, are also a good option for pelvic reconstruction [19]. However, their elevation is challenging in the lithotomy position, and subcutaneous tunneling is very tedious in the irradiated inguinal-perineal tissue, resulting in an inefficient surgical process. Due to these drawbacks, we suggest using gluteus-based flaps as the first-line choice, especially in recurrent, irradiated pelvic ablation cases. The advantages and disadvantages of various reconstructive methods are outlined in Table 2.
Cases 8 and 9 highlight the importance of local wound control by the reconstructive surgeon. Control of wound breakdown or fistula formation after colorectal surgery poses a high risk for wound superinfection and progression into Fournier gangrene [20]. Long-term complications usually present as a small skin breakdown, which is usually an opening from the fistulous tract originating from the deep tissue layer. Therefore, the overall extent and severity of the wound are mostly obscured and are actually much more serious than the external presentation. Considering the deteriorated condition of the patient in case 8, we decided to apply serial NPWT. As seen in our cases, we believe NPWT and appropriate antibiotics are very effective in obliterating narrow and deep fistulous wounds. Additionally, case 9 shows an example of a proactive attempt to prevent wound dehiscence by using NPWT in an immediate setting. In this situation, NPWT is a useful adjunct not only in treatment but also for the prevention of wound breakdown.
In conclusion, advanced perioperative care and the development of surgical techniques allow oncologic surgeons to perform more extensive surgical tumor ablations. Patients who were once considered inoperable can seek surgical treatment and may benefit from reduced need for chemoradiation and, hopefully, attain a higher quality of life during treatment. However, the increased extensiveness of the pelvic ablative procedure involves empty pelvis syndrome. Therefore, compensating for volume and skin with tissue flap is needed. The authors recommend flaps from the gluteal region as the first choice for this specific reconstructive demand because gluteus-based flaps can provide various tissue types (skin, subcutaneous fat, and muscle) and help maintain a high degree of freedom in intraoperative patient positioning, either prone or supine. Further accumulation of patient data may provide a rationale for reconstructive decisions in practice, ultimately improving patient outcomes.
Notes
No potential conflict of interest relevant to this article was reported.