Consistent Reconstruction of Sacrococcygeal Pressure Ulcers using Modification of En Bloc Sliding Gluteus Maximus Myocutaneous Flap Technique
Article information
Abstract
Background
The en bloc sliding gluteus maximus myocutaneous flap was introduced to preserve the vasculature, muscular integrity, sensory innervation, and normal gluteal contour with a midline scar in sacrococcygeal pressure ulcer reconstruction. However, its critical disadvantages include incomplete detachment of the origin of the gluteus maximus and central tension of the closed wound due to round ulcer excision. Therefore, we reviewed the surgical anatomy and applied modifications to achieve sufficient flap mobilization and to decrease complications.
Methods
After fusiform or rocket-shaped ulcer excision, submuscular flap elevation was initiated by completely detaching the origin of the gluteus maximus, including the posterior iliac crest, followed by comprehensive lateral submuscular dissection in the gluteal space while preserving the neurovascular pedicles. Bony protrusions were tangentially resected from the lower sacrum and upper coccyx. After en bloc medial advancement of the bilateral flaps, defects were closed in layers, with muscle ligament fixation at the midline.
Results
Twenty-nine patients underwent surgery for sacrococcygeal pressure ulcers (primary, n=22; recurrent, n=7). Transverse width of the excised ulcers was 5–12 cm (final defect, 7–15 cm). During the follow-up period (6 months to 7 years), no early postoperative complications or late aesthetic or functional discomfort occurred; however, intermittent skin sloughing occurred in four cases and one coccygeal sore recurrence occurred. The recurrent ulcer was treated using the same surgical method, with no recurrence after 2 years.
Conclusion
This modification can be successfully used for the reconstruction of primary and recurrent sacrococcygeal pressure ulcers.
Introduction
Surgical reconstruction of pressure ulcers generally involves ulcer excision, radical debridement of the involved bone, and coverage using large regional pedicle flaps with sufficient blood supply and soft tissue bulk [1-3]. The most critical step for successful reconstruction is appropriate flap selection based on reliability, reusability, minimal adjacent tissue damage, and preservation of future surgical options [2,3]. Modifications of the gluteus maximus myocutaneous (GMMC) flap have a high success rate for coverage of sacrococcygeal ulcer defects [1-3].
In 1990, Ramirez [4] described a bilateral en bloc sliding advancement GMMC flap for sacrococcygeal wound reconstruction. The sequential procedure of this technique includes ulcer excision in a round shape accompanied by appropriate ostectomies when necessary, detachment of the gluteus maximus (GM) origin (lower 3/4), and medial advancement of the GMMC flap without gluteal skin islands or lateral subcutaneous dissection. This technique is simple, minimally invasive, reusable, and reliable, thereby allowing preservation of the gluteal vasculature, muscular integrity, sensory innervation, and external gluteal contour; it also leaves an inconspicuous midline scar. Therefore, we originally believed that this may be the most reliable flap to satisfy all requirements for sacrococcygeal pressure ulcer reconstruction.
However, in our experience, it has some critical disadvantages: difficulty in both approaching anatomical structures to completely detach the GM origin without neurovascular damage and in achieving tension-free closure of the skin and muscle at the center of the midline wound. We believe that this may be caused by round-shaped ulcer excision, which provides a relatively narrow operative field, thus leading to incomplete or non-detachment of the GM origin from the posterior iliac crest, as previously described [4]; this results in insufficient medial advancement of the GM origin. Additionally, it induces intensive tension at the wound margins at the level of the maximal transverse width upon midline closure of the round wound. This frequently results in wound problems or midline closure failure in large ulcers, particularly in recurrent cases. However, solution-oriented modifications and refinements have not been reported.
To establish a wide surgical field to safely approach the sacrogluteal structures and to achieve even tension distribution when closing the midline muscle and skin wound, we refined the original technique [4] with fusiform or rocket-shaped ulcer excision, additional detachment of the GM origin, tangential dorsal ostectomy of all sacrococcygeal bony protrusions which might have contributed to the pressure injury, and muscle ligament fixation during midline closure.
This study aimed to review the surgical anatomy of the sacrogluteal area and to describe the modification of the bilateral en bloc sliding advancement GMMC flap technique with surgical refinements that facilitate consistent and safe reconstruction of sacrococcygeal pressure ulcers.
Methods
This retrospective study was approved by the Institutional Review Board of Kyung Hee University Hospital (No. KHUH 2022-10-013) and was performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants. Twenty-nine patients (17 men and 12 women) aged 21–73 years (mean 50.6 years) underwent reconstructive surgery for stage 4 sacrococcygeal pressure ulcers with bone exposure (22 primary and 7 recurrent) from 2011 to 2019 (Table 1). Among these patients, six were ambulatory and 23 were non-ambulatory.
Surgical anatomy
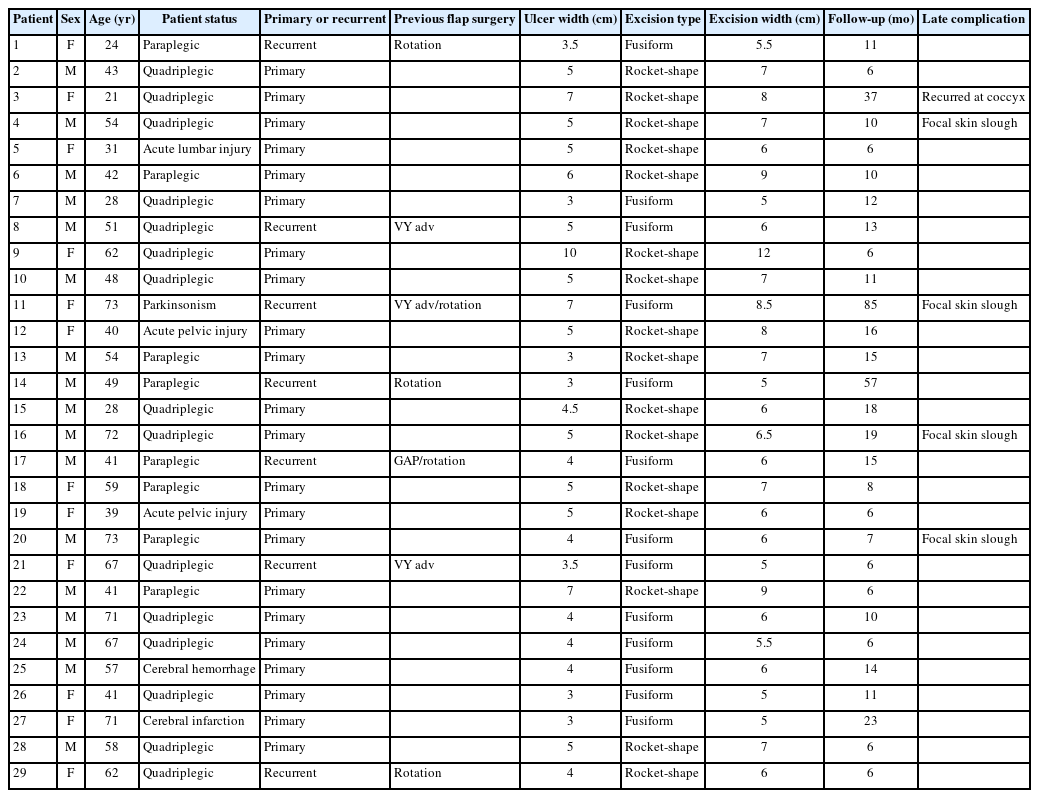
On the sacrum and coccyx, the dorsal bony prominences that most commonly provoke pressure ulcer formation consist of the lower median (MSC) and intermediate sacral crests (ISC), the sacral (SH) and coccygeal horns (CH), the first coccygeal body, and the lateral sacral crests (LSC) (blue dotted line, Fig. 1A) [5-7]. Four posterior sacral foramina (PSF) are located between the ISC and LSC. The sacral hiatus is the caudal opening of the sacral canal on the posterior midline of the fifth and, occasionally, fourth sacral vertebra.
Surgical anatomy of the sacrococcygeal and gluteal regions. (A) The skeletal structures of bones and ligaments. The blue dotted lines indicate the dorsal bony prominences of the sacrum and coccyx that can provoke pressure ulcers. The red dotted line indicates the gluteus maximus origin attaching to the bony structures and ligaments. (B) The muscular and neurovascular structures. PSIS, posterior superior iliac spine; PSF, posterior sacral foramina; MSC, median sacral crest; ISC, intermediate sacral crest; LSC, lateral sacral crest; GT, greater trochanter; SH, sacral horn; CH, coccygeal horn; Sh, sacral hiatus; IT, ischial tuberosity; LPSL, long posterior sacroiliac ligament; ISL, interspinous ligament; SCL, sacrococcygeal ligament; STL, sacrotuberous ligament; Gm, gluteus medius; GM, gluteus maximus; SGA, superior gluteal artery; P, piriformis; IGN, inferior gluteal nerve; IGA, inferior gluteal artery; SN, sciatic nerve; PFCN, posterior femoral cutaneous nerve; LCN, lateral cluneal nerve; SCN, superior cluneal nerve; MCN, medial (middle) cluneal nerve; ICN, inferior cluneal nerve.
The long posterior sacroiliac ligament (LPSL) courses downward from the posterior superior iliac spine (PSIS), attaching to the LSC and the sacrotuberous ligament (STL) (Fig. 1A). The STL courses from the dorsolateral portion of the lower sacrum and the upper coccyx to the ischial tuberosity (IT). The lateral edges of the ligaments form the posterior boundary of the gluteal space.
The GM originates from the posterior iliac crest, including the PSIS, erector spinae aponeurosis, lower lateral sacrum (SH and ISC to LSC), CH, STL and sacroiliac ligaments (red dotted line, Fig. 1A). It inserts into the greater trochanter (GT) and gluteal tuberosity of the femur and is innervated by the inferior gluteal nerve (IGN) (Fig. 1B) [5-7]. The piriformis originates from the anterior surface of the sacrum (S2–S4) and inserts into the upper border of the GT.
The arterial supply to the gluteal area primarily consists of the superior (SGA) and inferior gluteal arteries (IGA) that enter the gluteal space, accompanied by the superior gluteal nerve and IGN above and below the piriformis together forming a 3- to 4-cm vascular pedicle that obliquely follows the deep surface of the GM (Fig. 1B) [5-11]. The IGA is located 5 cm from the sacral edge [8,9] or 1 cm from the superior edge of the STL [9]. On the buttocks, it is usually located halfway [10] or two-fifths [11] along the line from the PSIS to the IT. The SGA is generally located 5 cm from the sacral edge [8], 3 cm cephalad to the IGA [9], one-third along the line from the PSIS to the GT [10], or 1 cm medial to its point [11]. The arterial supply to the sacral region consists of the terminal branches of the lateral sacral arteries that exit the PSF.
The cutaneous sensory nerves in the sacral region consist of the branches of the dorsal sacral rami exiting the PSF. In the gluteal region (Fig. 1B) [6,7], the medial cluneal nerves (MCN) innervate the medial gluteal area as branches of the dorsal sacral rami. The superior cluneal nerves (SCN) arise from the dorsal lumbar rami and innervate the superior aspect as the leading distributor. The inferior cluneal nerves (ICN) originate from the posterior femoral cutaneous nerve and innervate the inferior aspect. The lateral cluneal nerve originates from the iliohypogastric nerve and innervates the lateral aspect.
Surgical procedure
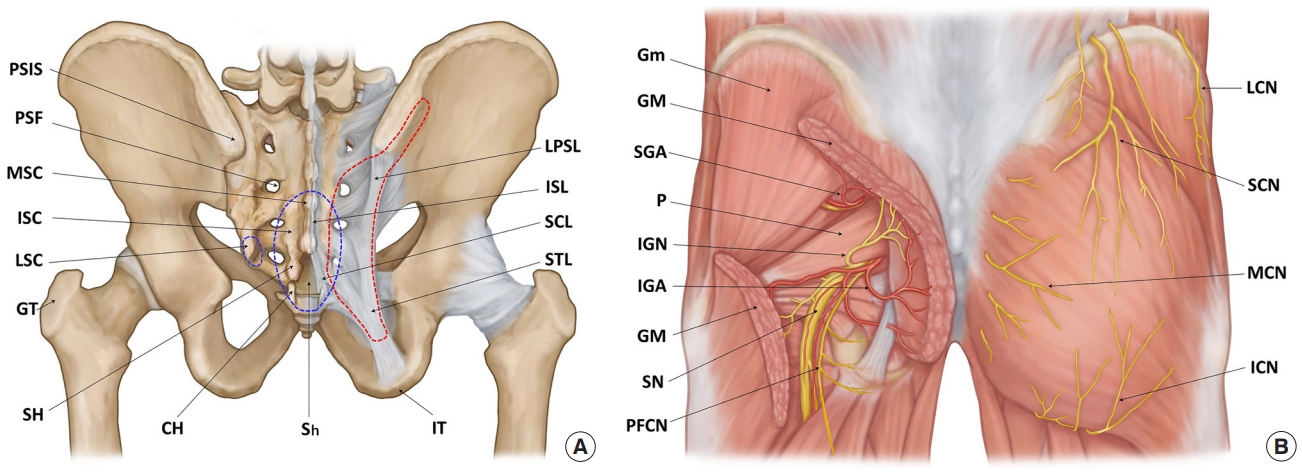
The surgical procedure for our modified bilateral en bloc sliding advancement GMMC flap technique consisted of fusiform or rocket-shaped ulcer excision, submuscular flap elevation with complete detachment of the GM origin, tangential dorsal ostectomy, and flap coverage by midline closure of the muscle and skin with muscle ligament fixation (Fig. 2).
Modified surgical procedure of the bilateral en bloc advancement flap. (A) Design of cephalocaudal ulcer excision. (B) After en bloc ulcer excision, the gluteus maximus origin was completely detached from the sacrum, coccyx, long posterior sacroiliac ligament, sacrotuberous ligament, and posterior iliac crest. Submuscular dissection of the gluteal space was performed, thus preserving the superior and inferior gluteal pedicles on both sides, then followed by tangential dorsal ostectomy of the bony protrusions on the sacrum and coccyx. (C) The medial margins of the gluteus maximus were medially advanced and closed with muscle ligament fixation in the midline. (D) Immediate postoperative view after skin closure.
The skin incision line for ulcer excision was designed cephalocaudally in a fusiform or rocket shape, passing approximately 1 cm lateral to the ulcer margin at the level of the maximal transverse width (Figs. 2A, 3A). This excision shape facilitated the detachment of the GM origin from the posterior iliac crest and the distribution of tension in midline muscle and skin closure. First, a fusiform excision line was designed (Fig. 3A). The cephalic end of the fusiform which was first excised was located at or above the level of the PSIS. The caudal end of the fusiform was located along the intergluteal cleft, but did not cross the tip of the coccyx. In cases where the ulcer invaded the anal region because of its large size or lower location, the caudal portion of the fusiform was converted into a rocket shape with two wings on the medial buttocks lateral to the anus to avoid perianal injury and wound contamination from the anus (Fig. 2A). When necessary, the SGA and IGA exiting the gluteal space were marked on the skin surface [10,11]. After skin incision, en bloc ulcer excision was performed, including the bursa, unstable scar tissue, and normal skin above and below the ulcer. In rocket-shaped excisions, the skin of the rocket wings was usually excised during final wound closure.

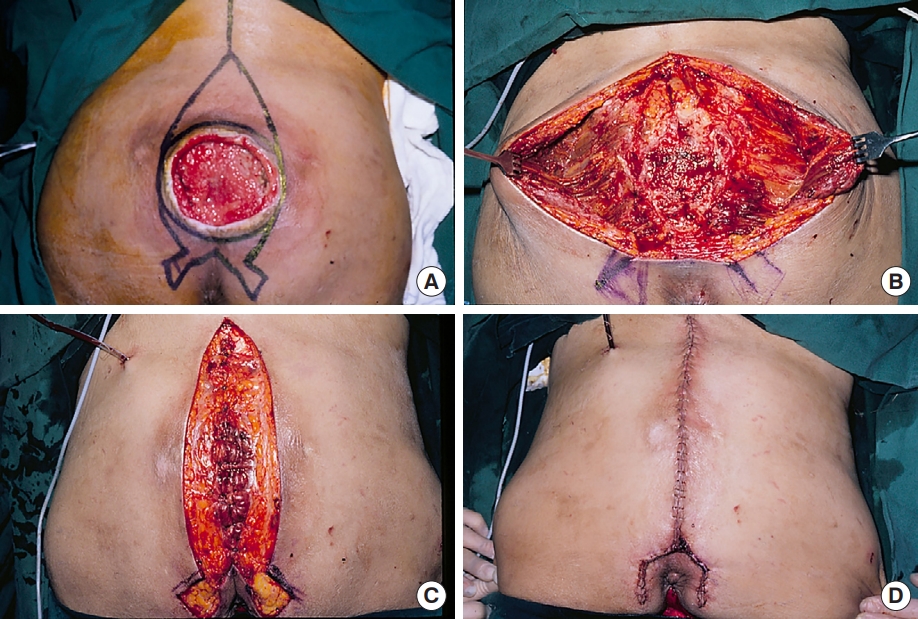
Cases of sacrococcygeal pressure ulcer reconstruction. (A) Recurrent pressure ulcer in a 73-year-old woman with Parkinson’s disease who had previously undergone flap surgery twice. A fusiform ulcer excision with a width of 8.5 cm was designed. (B) Image taken 7 years postoperatively. (C) Primary pressure ulcer extending to the medial buttocks in a 21-year-old quadriplegic woman. A rocket-shaped ulcer excision with a width of 8 cm was designed. (D) Image taken 4 weeks postoperatively. (E) Primary pressure ulcer in a 42-year-old paraplegic man. A rocket-shaped ulcer excision with a width of 9 cm was designed. (F) Image taken 2 months postoperatively. (G) Primary pressure ulcer in a 40-year-old ambulatory woman with acute lumbar spine injury and diabetes mellitus. A rocket-shaped ulcer excision with a width of 8 cm was designed. (H) Image taken 16 months postoperatively.
Submuscular flap elevation was initiated by detaching the periosteal or ligamentous origin of the GM from the lower sacrum and upper coccyx (Fig. 2B). The branches of the lateral sacral artery and dorsal sacral nerve (MCN) exiting the PSF were cauterized. Muscular detachment continued latero-inferiorly along the LPSL and STL until the gluteal space was identified, considering the location of the inferior gluteal neurovascular pedicle. The perforating vessel exiting the distal portion of the STL was cauterized. Additional detachment of the muscle origin was performed along the posterior iliac crest, including the PSIS.
For flap elevation in the gluteal space, blunt submuscular dissection was performed laterally; a thin layer of muscle fibers or submuscular fascia was retained on the bed to avoid dead space (Fig. 2B). The SGA and IGA were identified by gently dissecting the established locations. While protecting the pedicles, blunt submuscular dissection was continued laterally, up to the midline of the buttocks.
Subsequently, all bony prominences on the dorsum of the lower sacrum and upper coccyx, including the MSC, ISC, SH, CH and the first coccygeal body, were tangentially resected with a reciprocating saw and grinding burr until the dorsal surface was even and curved without any sharp bony edges (Fig. 2B).
Tension-free defect closure began by confirming sufficient medial advancement of the flaps across the midline. When flap advancement was insufficient, additional muscular and fascial detachment was performed on the posterior iliac crest and gluteus medius superiorly, and on the STL and ischioanal fat tissue inferiorly. A negative-pressure suction drain was placed along the parasacral space in a U-shaped manner. Next, both GM muscles were securely sutured in two layers using 2-0 absorbable sutures at the midline. The muscles in the deep layer were simultaneously sutured to the remaining interspinous ligaments or the aponeurosis of the erector spinae superiorly, and to the posterior sacrococcygeal and intercoccygeal ligaments inferiorly. Muscle ligament fixation provided a suitable bony attachment for the GM origin and prevented muscle detachment from the sacrum and submuscular dead space. The subcutaneous fascia was approximated using 4-0 nylon sutures, followed by skin closure using 5-0 vicryl and nylon sutures (Fig. 2D). The skin on the rocket wings was excised and trimmed to avoid dog ears on the medial buttocks. A mild compressive dressing with surgical pads and elastic bandages was applied.
All patients were placed on the bed in a prone position with extension of the hip joint for 2 weeks, with temporary lateral decubitus position permitted for a few hours at a time when necessary. After 2 weeks, the patients were then allowed to adopt either a supine position with a supportive cushion applied to the lumbar area to prevent flap compression or a lateral decubitus position at leisure; they were ambulated carefully in a wheelchair for a short time. Ambulatory patients were allowed to ambulate carefully in a standing position and go to the bathroom after 7 days. After 3 weeks, the patients were allowed to remain in any position on the bed and in a sitting position for wheelchair ambulation.
Results
The initial ulcers were 3–10 cm in maximal transverse width; the ulcer excisions were 5–12 cm; and the final skin defects after ulcer excision were 7–15 cm, including recurrent ulcers in which the ulcer excision was up to 8.5 cm (final defect, up to 13 cm) (Fig. 3). Cephalocaudal ulcer excision shape was fusiform and rocket-shaped in 13 and 16 patients, respectively. The mean postoperative hospitalization period was 21.3 days (range, 18–26 days) for non-ambulatory patients and 13.5 days (range, 12–15 days) for ambulatory patients.
All sacrococcygeal pressure ulcers healed successfully without early postoperative complications such as hematoma, seroma, infection, delayed healing, or wound dehiscence. During the follow-up periods (6 months to 7 years, mean 15.8 months), no significant aesthetic or functional problems occurred in the ambulatory patients, including gluteal disfigurement, sensory impairment, or gait disturbance (Fig. 3). Four non-ambulatory patients experienced intermittent skin sloughing in the coccygeal area, which however healed spontaneously (Fig. 3A and B). One young quadriplegic female patient (Fig. 3C and D) experienced a small coccygeal recurrence 13 months postoperatively. This recurrent ulcer was treated using the same surgical procedure, including tangential dorsal ostectomy of the first coccygeal segment, which was insufficiently resected in the previous surgery; this resulted in no subsequent recurrence during the 2-year follow-up period.
Discussion
Sacrococcygeal pressure sores result from ischemic injury inflicted on skin and subcutaneous tissue sandwiched between external weight bearing forces and dorsal bony protrusions of the lower sacrum and upper coccyx. Therefore, the principle of surgical reconstruction is excision of the ischemic ulcer tissue, radical resection of the protruding bony portion and wound coverage using a reliable flap with sufficient blood supply and tissue bulkiness [1-4]. For successful reconstruction, it is important to select an appropriate flap based on reliable bulk, blood supply and sensation, reusability and preservation of future surgical options with minimal adjacent tissue damage [2].
The V-Y advancement GMMC flap with large triangular islands has long been used as the primary or secondary flap choice for covering large sacral or sacrococcygeal pressure sore defects [1-3]. This technique has several advantages: ability to cover large defects with ample tissue bulk, excellent blood supply, and reusability through re-advancement of the same flaps. However, a full-layer skin-fat incision of the gluteal area as well as wide lateral subcutaneous dissection is required for large skin islands. Consequently, this procedure completely transects sensory innervation of the SCN and ICN and severs the perforating arteries of the SGA and IGA in the surrounding gluteal region, resulting in decreased flap sensation, limitation of future flap selection even if re-advancement of the V-Y GMMC flap itself is an option, and gluteal disfigurement due to large Y-shaped scars. Thus, it is questionable whether this flap is consistently appropriate for pressure ulcers less than 12 cm in transverse width, recurrent ulcers or patients requiring sacro-gluteal sensation. Recently, the gluteal artery perforator (GAP) flap and its modifications are commonly used in sacral ulcers [12-14]. However, this technique also has several disadvantages: severance of gluteal cutaneous nerves resulting in considerable decrease of flap sensation, wide supra-muscular dissection for flap elevation and isolation of the main gluteal artery which may limit future flap selection for recurrent ulcer reconstruction, and gluteal donor site closure which can lead to gluteal disfiguration.
The original en bloc sliding advancement GMMC flap was elevated only by wide submuscular dissection with detachment of the GM origin without any gluteal skin incision or subcutaneous dissection. It manifested the role of a true sensate flap innervated from the SCN and ICN. This technique preserved the gluteal vasculature, muscle integrity, flap sensation, normal gluteal skin, and external contours leaving a midline scar. It also preserved all future flap options. Therefore, it was a useful option to reconstruct primary and recurrent ulcers in both ambulatory and non-ambulatory patients.
However, the original surgical procedure consisted of round-shaped ulcer excision, submuscular dissection with incomplete detachment of the GM origin, and midline closure of the round defect associated with dog-ear correction in the upper and lower portions [4]. Most sacrococcygeal pressure ulcers were located in the lower sacrum or upper coccyx. Therefore, round ulcer excision only enabled a narrow operative field which made detachment of the GM origin along the posterior iliac crest difficult, thereby resulting in limited muscular mobilization especially on the upper sacrum. Midline closure of round-shaped excision defects also resulted in maximal horizontal tension at the center area and dog-ear formation with subcutaneous dead space on the upper sacrum and lower coccyx, which led to an increased risk of wound problems such as delayed healing, wound dehiscence, and closure failure in large ulcers, and also intergluteal cleft distortion.
In our surgical refinements, fusiform ulcer excision allowed a wider operative field, with a more straightforward approach for optimal detachment of the GM origin along the posterior iliac crest, thereby decreasing the stepwise elevation of tension toward the midline muscle-skin closure site. It also prevented the formation of dog-ear deformities and subcutaneous dead spaces in the upper and lower portions of the midline closure wound. However, in large pressure ulcers located on or caudal to the sacrococcygeal junction, the caudal end of the fusiform excision frequently invaded the anal region, thus resulting in an increased risk of perianal injury and wound contamination. When the design of the lower portion of the fusiform excision was altered to avoid anal intrusion, it inevitably appeared as a horizontal band at the intergluteal cleft between the coccyx and anus due to a dog-ear deformity after wound closure, thereby resulting in the formation of a deep valley just above the anus and causing difficulties in post-defecation anal care.
The rocket-shaped excision avoided making skin incisions in the anal region while successfully excising pressure ulcers extending into the medial buttock and provided sufficient medial advancement of the flaps at the sacrococcygeal joint level. Its closed wound line ran along the medial margin of the gluteal region peripheral to the anal area, thus avoiding intergluteal cleft distortion. Therefore, it was usually used for large pressure ulcers encroaching near the anus or on the medial buttocks (Figs. 2, 3C–H).
Complete detachment of the origin of the GM may be essential for greater muscular mobilization and flap advancement. However, in the original en bloc sliding advancement GMMC flap technique [4], the GM origin was detached from the sacrum, coccyx, STL, and LPSL only in the middle and lower portions, thereby resulting in limited muscular mobilization. Therefore, in our modification, the upper portion of the GM was detached along the posterior iliac crest, including the PSIS. Complete detachment of the GM origin provided sufficient muscle and flap mobilization, thus allowing tension-free closure at the midline.
The medial borders of the GM were fixed to the remaining ligaments or aponeurosis of the upper sacrum and coccyx during midline muscle closure; this was to reorient the muscle origins and prevent postoperative muscular displacement or submuscular dead space formation. We believe that our modified procedures of cephalocaudal ulcer excision and additional detachment of the GM origin along the posterior iliac crest as well as midline muscle ligament fixation prevented the occurrence of any early postoperative complications in all of our patients.
Some concerns have been raised about muscle weakness or gait disturbance due to complete detachment and medial advancement of both GM origins, especially in ambulatory patients. However, Ramirez et al. [9] described the V-Y advancement GMMC flap technique in ambulatory patients with sacral defects, in which bilateral GM origins were completely detached and medially advanced, as in our technique. They evaluated gluteal muscle function using step-climbing evaluation and electromyography between 8 months and 2 years after surgery and demonstrated no detectable muscle weakness or abnormal electromyographic results. Our opinion was that medial advancement of the GM origins may have induced GM muscle tightening, which prevented the development of muscle weakness. In our study, there were also no complaints of gait problem in ambulatory patients.
The main bony prominences causing sacrococcygeal pressure ulcers are the lower MSC and ISC, SH, CH, first coccygeal body and the LSC. The site of recurrence and intermittent skin sloughing during the follow-up period was the first coccygeal segment, which had presumably been insufficiently resected during the initial surgery. Therefore, tangential dorsal ostectomy of the bony prominences of the first coccygeal segment and lower sacrum may be essential for preventing wound dehiscence, recurrence or late wound complications.
The key advantage of this technique may be preservation of main gluteal vasculature while preventing damage to the gluteal tissues. This permits preservation of all future surgical options, including V-Y GMMC and GAP flaps as well as reusing this flap itself. In our series, one case of coccygeal ulcer recurred after surgery using this technique and was subsequently reconstructed by re-use of the same technique, resulting in no recurrence for 2 years. If the same patient has another recurrence in the future, a V-Y GMMC or GAP flap may be considered at that time as the choice for reconstruction. Furthermore, this technique could also be used to reconstruct recurrent pressure ulcers of patients who have already undergone first or second surgeries using V-Y GMMC, GAP or rotation flaps.
A limitation of this technique was the difficulty closing ulcers with a maximal transverse width of >12 cm. In such unexpected cases where the large final defect was not able to be closed by our modified technique, we immediately converted to the V-Y advancement GMMC flap technique. Additionally, this study did not include objective long-term examinations such as computed tomography scans or ultrasonography for evaluating changes in muscle or subcutaneous tissue thickness, despite there being no complaints of gait problems in the ambulatory patients.
In conclusion, the bilateral en bloc sliding advancement GMMC flap technique for reconstructing sacrococcygeal pressure ulcers was designed to preserve soft tissue connections, vasculature, muscle integrity, sensory innervation, and normal gluteal contours with a final midline scar. Our modification with fusiform or rocket-shaped ulcer excision, complete detachment of the GM origin, tangential dorsal ostectomy of the sacrum and coccyx, and muscle ligament fixation during muscle closure allowed for consistent and safe reconstruction of sacrococcygeal ulcers, with successful closure of ulcer excisions of up to 12 cm in width in primary cases and up to 8.5 cm in recurrent cases. This modification, while providing a sensate flap, was minimally invasive, reusable, and sufficiently reliable to satisfy flap selection requirements including preservation of all future surgical options. Therefore, we consider this modification as the primary option for reconstructing primary and recurrent sacrococcygeal pressure ulcers in both ambulatory and non-ambulatory patients.
Notes
No potential conflict of interest relevant to this article was reported.