The Effects of Massage with Topical Agents (Mepiform Ultra Scar Gel or Scarnos Gel) on Scar Tissue Thickness and Fibroblast Proliferation in Rats
Article information
Abstract
Background
Keloid and hypertrophic scars are prominent scars that are excessively repaired with upregulated synthesis, deposition, and accumulation of collagen. Topical agents are used to reduce inflammation and fibrotic changes via reduced transepithelial water loss. Increased mechanical pressure applied through scar massage can accelerate scar maturation by inducing fibroblast proliferation, which enhances the remodeling of connective tissue matrices and collagen degradation.
Methods
This study comparatively analyzed the effectiveness of topical agents applied to post-burn wounds on the dorsal surface of Sprague-Dawley rats through twice-daily massaging. Postoperative histological analysis of the tissues was performed after surgical en bloc removal of the treatment area at 4, 10, and 16 weeks.
Results
Histological analyses revealed larger amounts of fibroblasts in Mepiform and Scarnos gel-treated tissue than in Vaseline-treated tissue. Granulation was prevented in scars treated with the topical agents.
Conclusion
Mepiform Ultra scar gel and Scarnos gel, accompanied by massaging, may be effective anti-scarring topical agents to alleviate contact burn scars in Sprague-Dawley rats.
Introduction
Wound healing involves the inflammatory phase, proliferative phase, and remodeling phase, among which there is some overlapping [1]. The inflammatory phase begins the moment a wound is inflicted. Activating the coagulation cascade induces release of cytokines that stimulate the chemotaxis of unspecific immune cells such as macrophages and neutrophils into the wound for initial cellular wound debridement [2]. Meanwhile, the proliferative phase is associated with recovery of the epithelium and granulation tissue formation, angiogenesis, wound contracture, and deposition of new extracellular matrix (ECM) 48 to 72 hours after wound initiation [3,4]. In this phase, the new ECM that bridges gaps in the tissue consists of collagen, fibroblasts, and small vessels, which mostly contribute to scar formation [3,4]. ECM synthesis and degradation are regulated by increased collagen synthesis and decreased fibroblast proliferation [3].
Scars can appear in various forms ranging from mature linear scars to hypertrophic scars and keloids, which are abnormally excessive scars. The incidence of excessive scars formed after surgery or trauma is estimated to be approximately 15% with a particularly high prevalence following burns [5,6]. Keloid and hypertrophic scars are excessively formed through upregulated collagen synthesis, deposition, and accumulation. The contracture of these scars leads to pain and itching, which may require continuous dressing treatment because of the formation of bullae, followed by ulceration. Additionally, repeated ulceration can induce the formation of Marjolin ulcer and squamous cell carcinoma. Keloids can invade adjacent normal tissues across the boundaries of the original wound [5]. Moreover, protruding or long-lasting scars on the visible parts of the skin can affect personal confidence, leading to problems in daily life.
Various techniques have been employed to remove scars, such as laser ablation or surgical excision; preventing the occurrence of protruding scars during the remodeling phase immediately after the proliferative phase of scar formation can be a more cost-effective and noninvasive treatment strategy. Therefore, application of topical agents or gel sheets is recommended especially for patients with a history of keloids or hypertrophic scars to prevent additional keloid or hypertrophic scarring.
Topical agents are used to reduce inflammation and fibrotic changes via decreased transepithelial water loss (TEWL). Increasing TEWL elevates the expression of proinflammatory cytokines and chemokines, such as S100A8/A9, which play a role in acute or chronic inflammation and result in fibroblast activation and excessive collagen production [7].
Moreover, increased mechanical loading with scar massage can accelerate scar maturation by inducing fibroblast proliferation, which enhances the remodeling of connective tissue matrices and the degradation of collagen synthesis. In other words, increased mechanical loading with scar massage promotes tissue turnover and regeneration, associated with synthesis and realignment of collagen, which are in turn induced by localized inflammation and increased perfusion [8]. Therefore, an increased abundance of fibroblasts is expected with a greater amount of tissue turnover.
Because patients often bring us a variety of silicone-based scar ointments they had been using, wanting to confirm their efficacy, it was necessary to verify whether certain silicone products were effective in preventing scars in order to inform the patients if they should continue using them. This included the scar-prevention effects of cyclopentasiloxane in Mepiform Ultra scar gel, which is generally included in personal care products rather than scar management agents. This study therefore aimed to compare the effects of Mepiform Ultra scar gel and the previously used topical agent Scarnos gel, which contains the most commonly used medical silicone in scar management products, applied through massage on post-burn wounds on the back of Sprague-Dawley rats.
Methods
Experimental animals
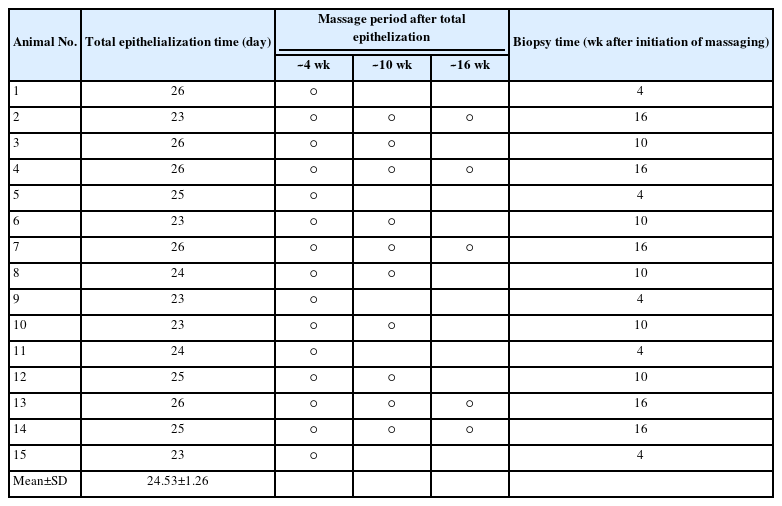
Fifteen 8-week-old male white Sprague-Dawley rats (300–320 g) were acclimatized over a 1-week period in the laboratory. A food pellet diet and water were provided until the end of the experiment. Owing to limitations in laboratory conditions, only three of the 15 rats were placed in solitary cages, and 12 rats were placed in groups of two per cage. All experiments (including the use of animals) were performed following protocols approved by the Institutional Animal Care and Use Committee of Wonkwang University, Iksan, Korea (IACUC No. WKU22-117).
Experimental materials
The following topical agents were used in the study: Mepiform Ultra scar gel (a transparent, low-viscosity gel containing the silicone ingredient, cyclopentasiloxane, Molnlycke Health Care); Scarnos gel (an opaque, non-viscous gel containing the silicone ingredient, polydimethylsiloxane, Henan Huibo Medical Co.); Vaseline (petroleum jelly, Anjin Pharm).
Wound induction
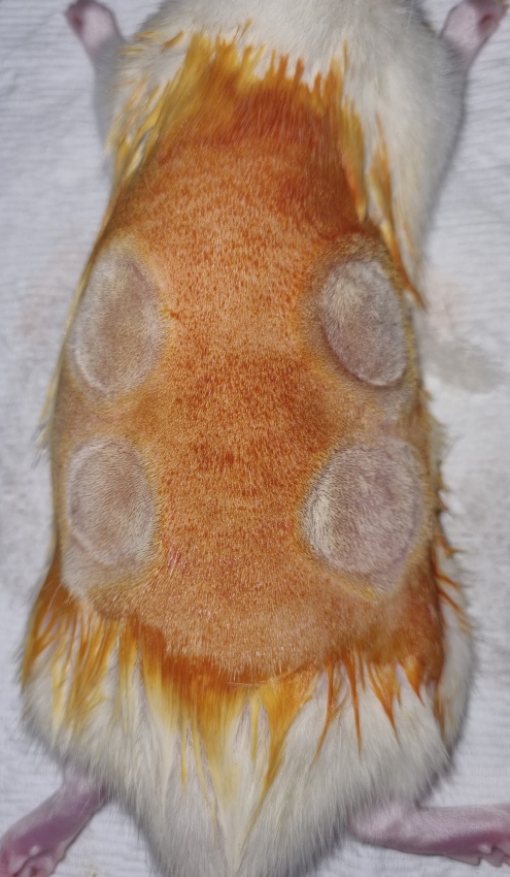
After a 1-week acclimatization period, isoflurane and sevoflurane (Baxter) were used for inhalation anesthesia. The surface of the dorsal area was disinfected with a 10% povidone-iodine and 70% alcohol solution and then shaved with an electric razor. Wounds were made by cauterizing for 15 seconds using an electrical soldering iron (tip diameter 20 mm; 200 ºC) in four quadrants on the back: upper left, upper right, lower right, and lower left; regular gaps of 1 cm were maintained between adjacent wounds to prevent interference among wounds (Fig. 1). The soldering iron was designed to measure and display the temperature and time (Fig. 2).
Wound care
After epithelization through daily dressing treatment, the wounds on each of the 15 rats were distributed into four groups: control, Vaseline, Mepiform Ultra, and Scarnos gel groups. The locations of the four treatment groups were randomly determined (Table 1). The control group received no scar treatment. The Vaseline group had Vaseline applied on post-burn scar sites twice daily without any massage, immediately after total epithelization. The Mepiform Ultra group was subject to bare finger massage using Mepiform Ultra for 5 minutes twice daily to prevent damage to the skin; massage was performed by a single person immediately after total epithelization. The same procedure was performed for the Scarnos gel group (Fig. 3). For histological analysis, surgical en bloc removal of the massage area of Sprague-Dawley rats was performed at 4, 10, and 16 weeks after initiation of massage. The overall experimental process is shown in Table 2.
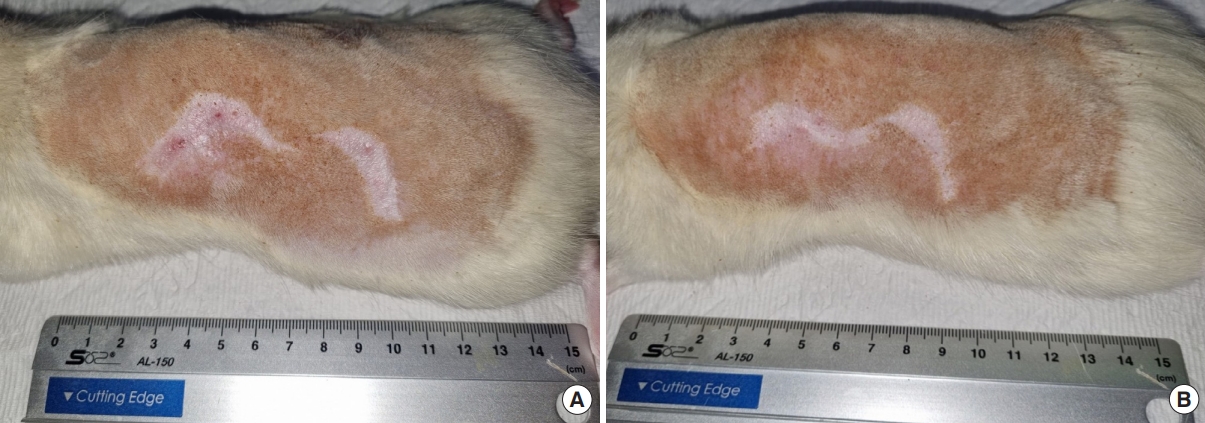
Wounds areas of rat no.14. (A) Photograph of week 10 after initiation of massaging. (B) Photograph of week 16 after initiation of massaging.
Wound biopsy
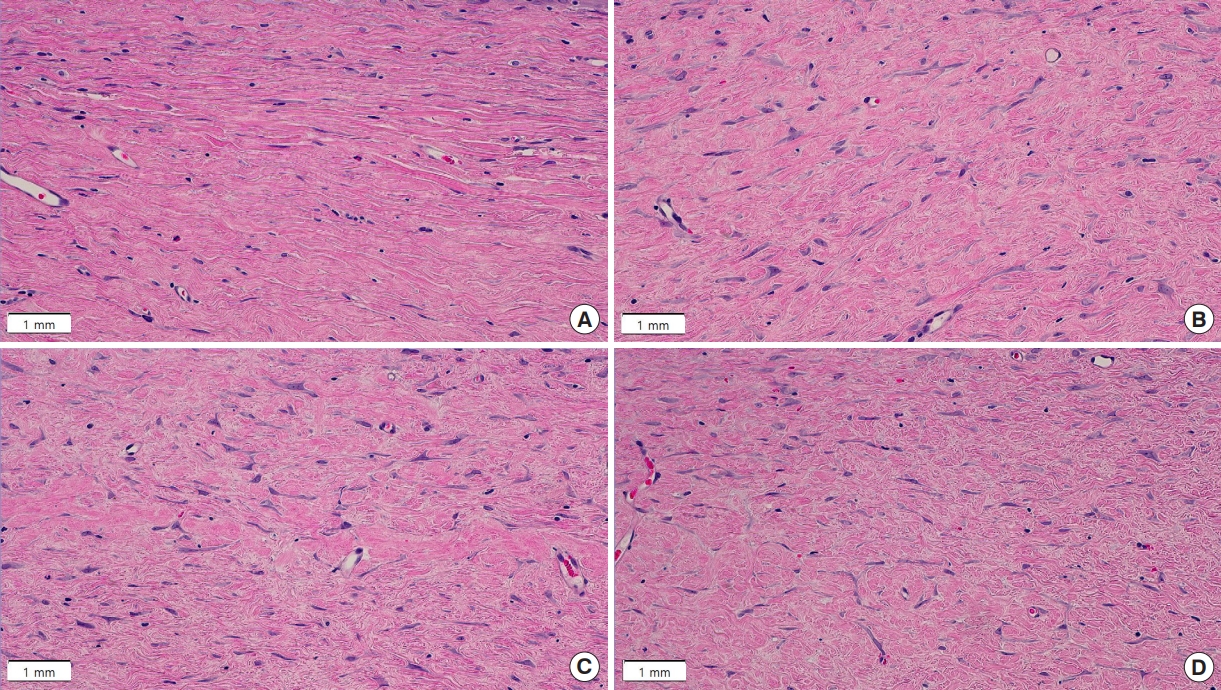
After gross epithelialization, five rats were selected for surgical en bloc removal of the massage areas including the scar tissues after 4, 10, and 16 weeks of massage. Tissue specimens collected from each rat were fixed through incubation in formalin solution at room temperature for more than 24 hours, prepared as paraffin block, cut into slices, and stained with hematoxylin and eosin and Masson’s trichrome (MT), conforming to standard protocols.
Histological evaluation and statistical analysis
The stained tissue samples were investigated under a microscope at ×40 and ×400 magnifications. The slides were scanned using a digital slide-scanning device, and ×40 magnification images were visualized using Olympus cellSens Standard software, version 3.2 (Olympus Corp.), while ×400 magnification were obtained with Image J software version 1.54d (National Institutes of Health).
Statistical results were expressed as mean±standard deviation. We compared the differences between the biopsy results from the two groups based on two parameters: scar tissue thickness detected at ×40 magnification and the number of fibroblasts under the scar tissue per field at ×400. Only the border of the scar area between the epidermal basement membrane and the panniculus carnosus was measured after MT staining to evaluate the scar area and the degree of granulation tissue formation [9]. The area of scar with granulation tissue was quantitatively measured in two different MT-stained specimens within the same wound [9].
The area with the highest abundance of fibroblasts was selected by two pathologists at ×400 magnification. Statistical differences in scar tissue thickness in the four groups were compared at the same time points, using the Kruskal-Wallis test and Mann-Whitney test to assess the continuous variables and multiple comparisons based on SPSS Statistics version 27 (IBM Corp.). The Kruskal-Wallis test was used to compare variables among three or more groups, while the Mann-Whitney test was used to compare continuous variables between two groups. Multiple comparisons of data between time points and between groups were performed using Tukey’s method. The level of significance was set at P<0.05.
Results
Gross epithelization time for each rat
The mean gross epithelialization time for each rat treated with quadrant locations for each treatment group is shown in Table 1. No rats were sacrificed during the experiment for reasons other than biopsy.
Histological evaluation
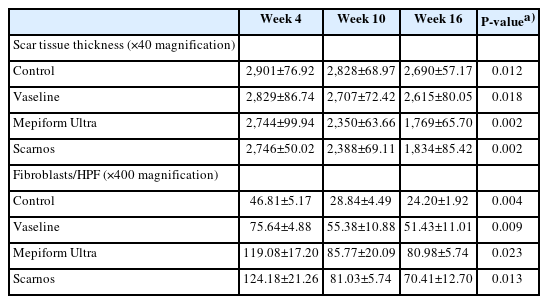
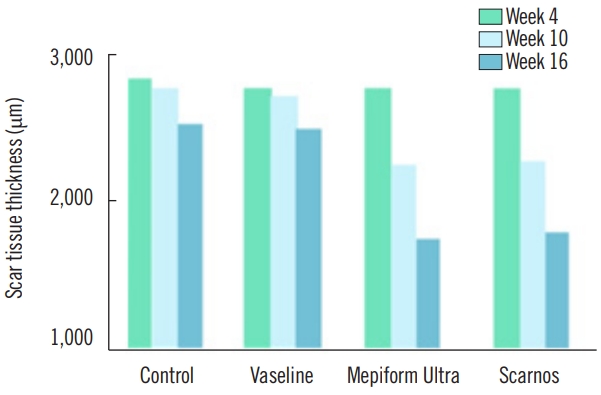
After 4 weeks of massage, the overall scar tissue in all four groups was thick. The mean scar tissue thickness in the control group, Vaseline group, Mepiform Ultra group, and Scarnos gel group were 2,901±76.92 μm, 2,829±86.74 μm, 2,744±99.94 μm, and 2,746±50.02 μm, respectively. Many fibroblasts were observed in the Mepiform Ultra and Scarnos gel groups that were massaged with topical agents; 119±17.20/high-power field (HPF) in Mepiform Ultra group and 124.2±21.26/HPF in Scarnos gel group (Table 3).
After 10 weeks of massage, significant differences were observed in the scar tissue thickness in the Mepiform Ultra and Scarnos gel groups, but no significant differences were observed in the control and Vaseline group; the mean scar tissue thickness in the control group, Vaseline group, Mepiform Ultra group, and Scarnos gel group were 2,828±68.97 μm, 2,707±72.42 μm, 2,350±63.66 μm, and 2,385±69.11 μm, respectively. Meanwhile, only the control group showed significant differences in the number of fibroblasts compared to the other groups; however, the number of fibroblasts in all four groups was reduced.
After 16 weeks of massage, significant differences were observed in the mean scar tissue thicknesses in the Mepiform Ultra and Scarnos gel groups but not in the control and Vaseline groups. The number of fibroblasts significantly decreased in all four groups. In particular, the number of fibroblasts in the control group was reduced by half. Large amounts of fibroblast proliferation were detected in the Mepiform Ultra and Scarnos gel groups, and the highest mean number of fibroblasts was in the Mepiform Ultra group (81±5.74/HPF in the Mepiform Ultra group and 70.4±12.70/HPF in the Scarnos gel group).
Scar tissue thickness
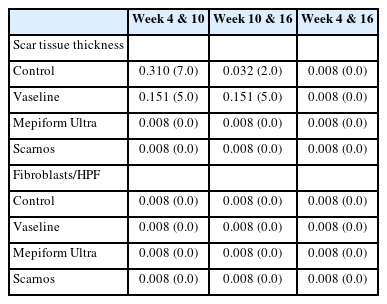
The mean±standard deviation of scar tissue thickness measured at weeks 4, 10, and 16 after initiation of massaging in each group are shown in Table 3. The changes in scar tissue thickness by groups are listed in Table 4. A comparison of scar tissue thickness by group for each week is shown in Table 5 and a comparison of scar tissue thickness by each week within the groups is shown in Table 6. No significant difference was detected in scar tissue thickness at week 4; the mean scar tissue thickness in the control group, Vaseline group, Mepiform Ultra group, and Scarnos group was 2,901±76.92 μm, 2,829±86.74 μm, 2,744±99.94 μm, and 2,746±50.02 μm (P>0.05), respectively (Table 3). However, there were significant differences in scar tissue thickness between the control group and the Mepiform Ultra and Scarnos gel groups at weeks 10 and 16 (P<0.05) (Table 5). Likewise, there were significant differences in scar tissue thickness between the Vaseline group and Mepiform Ultra and Scarnos gel groups. Nevertheless, there was no significant difference between control group and the Vaseline group (P>0.05) (Table 5). At week 16, scar tissue thickness was significantly decreased compared to that at week 4 in all four groups (P<0.05) (Table 6). Furthermore, there was no significant differences in thickness between the Mepiform Ultra and Scarnos gel groups during all periods (P>0.05) (Table 5, Figs. 4, 5).
Fibroblast frequency per microscopic field
The numbers of fibroblasts measured per microscopic field (×400) at weeks 4, 10, and 16 after initiation of massaging in each group are depicted in Table 3, and the changes in the numbers of fibroblasts by groups are listed in Table 4. A comparison of the numbers of fibroblasts by groups for each week is shown in Table 5, and a comparison of the numbers of fibroblasts by each week within groups is shown in Table 6. In all four groups, the number of fibroblasts per field decreased between weeks 4 and 10, and also between weeks 10 and 16 (P<0.05) (Table 6). The number of fibroblasts significantly differed between the control group and the Mepiform Ultra and Scarnos gel groups at weeks 4, 10, and 16, and also between the control group and Vaseline group (P<0.05) (Table 5). A significantly different number of fibroblasts was observed in the Vaseline group compared to the Mepiform Ultra group and Scarnos group at week 4 (P<0.05) (Table 5). However, no significant difference was detected between Vaseline group and Mepiform Ultra and Scarnos gel groups at week 10 (P>0.05) (Table 6). Significant differences were measured in the number of fibroblasts between the Vaseline group and the Mepiform Ultra and Scarnos gel groups at week 16 (P<0.05) (Table 5). No significant difference was observed between the Mepiform Ultra group and Scarnos gel group during all periods (P>0.05) (Table 5, Fig. 6).
Discussion
Outpatient clinic patients with a history of keloid or hypertrophic scars have an underlying fear of receiving surgical incisions, because they tend to think that all wounds inflicted on their bodies, including surgical incisions, will result in keloid or hypertrophic scars. The fact that simple surgical removal of keloids results in recurrence rates nearing 100% adds to their concerns [10]. Before surgery, such patients often inquire how long the scars will remain and when they can start using scar ointment, sheets, or laser ablation. Several methods such as steroid injections have been proposed to prevent hypertrophic scars and keloids in high-risk patients susceptible to environmental and genetic factors. Additionally, several studies were conducted on the treatment of hypertrophic scars and keloids, describing various approaches ranging from steroid tape and steroid injection to radiation therapy. However, early detection with close follow-up allows choosing the best time to start scar management, since observing how the scars develop is essential in attaining improved outcomes [11]. After that, selection of appropriate treatment for each scar must be followed.
We recommend scar ointment as a priority at our plastic surgery outpatient clinic. Most patients who want treatment for scars are concerned with body parts that are usually not concealed by clothing, and particularly areas where it is difficult to fix a silicone sheet, such as the face or hands [12]. Because of the discomfort caused by stiff silicone sheets covering the skin, patients usually prefer silicone gel over sheets; therefore, we focused on the effectiveness of silicone gel rather than silicone sheets.
The topical application of silicone gel is an effective method to prevent hypertrophic scarring [13]. Occlusion and hydration are expected when a silicone gel is applied to scars [14]. Silicone products provide an occlusive barrier and act as water reservoirs for the skin, preventing TEWL [14,15]. The positive effects of hydration downregulate the expression of genes, such as S100A8/A9, resulting in decreased tissue thickness in fibrosis [7]. Furthermore, pressure on scar tissue is assumed to cause microvessel hypoxia in scars and to support ECM-stimulated apoptosis [15]. The application of silicone gel through massage or pressure garments is often recommended by plastic surgeons. In hypertrophic scars, mechanical forces partially restore the ECM visible in normal scars and reduce α-smooth muscle actin-expressing myofibroblasts by apoptosis [16].
While polydimethylsiloxane of Scarnos gel is the most commonly used medical silicone applied to scars [17], the cyclopentasiloxane in Mepiform Ultra scar gel is used for personal care applications, not as a scar ointment [18]. According to the U.S. Food and Drug Administration, cyclopentasiloxane has been used in 2,459 cosmetic products [19]. The authors conduced this experiment because they believed that verifying the effectiveness of other ingredients rather than those already widely used in silicone gel or sheets would generate diversity in selecting a more suitable scar treatment agent for each patient.
According to this study, the use of Mepiform Ultra and Scarnos combined with massage is more effective in treating scars compared with Vaseline without massage. The anti-evaporation effects of Vaseline also alleviated scars compared the control group. Moreover, the Mepiform Ultra group exhibited less infiltration of inflammatory reactions and granulation tissue formation compared with the Scarnos group, though there was no significant difference between the two groups. Meanwhile, studies show that the use of topical silicone gel has no difference in efficacy compared to steroid cream [20]. In addition, many of the available “high evidence” clinical trials are at high risk of bias and it is unclear whether the effectiveness of silicone gels is comparable to silicone sheets and whether additional components present in many silicone gels may allow for scar healing [21]. Also, it is unknown whether the topical agents used in this study are effective for hypertrophic scar and keloid.
In this study, it was confirmed that scar tissue thickness significantly decreased in all groups over time; however, in the control group, the effect of time on scar thickness was weak until the 10th week. Also, the scar tissue thickness of the control group showed no significant difference from the Vaseline group at all time points. Meanwhile, there were significant differences between the control group and Mepiform Ultra and Scarnos groups at all time points.
Many variables were at play during this animal study. On post-burn day 1, one rat, upon observing the unfamiliar foam dressing attached to their bodies, partially removed the foam dressing of the paired rat placed in the same cage (rats 1 and 5); this must be considered as an unexpected variable. This shortcoming of the study could have been avoided had there been a sufficient number of cages to place one rat per cage.
In this experiment, massaging was to be consistently performed on the backs of all rats by the same researcher. However, some rats tried to avoid the massaging fingers. This factor interfered with the uniformity of massage throughout all selected dorsal areas on all rats, when high pressure applied to scars promotes a faster healing process than that promoted by light or moderate pressure [22].
Additionally, only three time points after initiation of massaging were used; more frequent investigations in smaller intervals could provide more precise information on the optimal time for effective application of topical agents. Longer-term research of at least half a year is required to determine whether silicone gel is effective in treating scars after 6 months [20].
The present histological studies revealed that scar massage using topical agents prevents granulation. Therefore, Mepiform Ultra and Scarnos scar gel with massaging can be considered effective anti-scarring topical agents to treat contact burn-induced scars in Sprague-Dawley rats.
Notes
Young Cheon Na is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.