Investigating Diabetic Foot Pathophysiology and Amputation Prevention Strategies through Behavioral Modification
Article information
Abstract
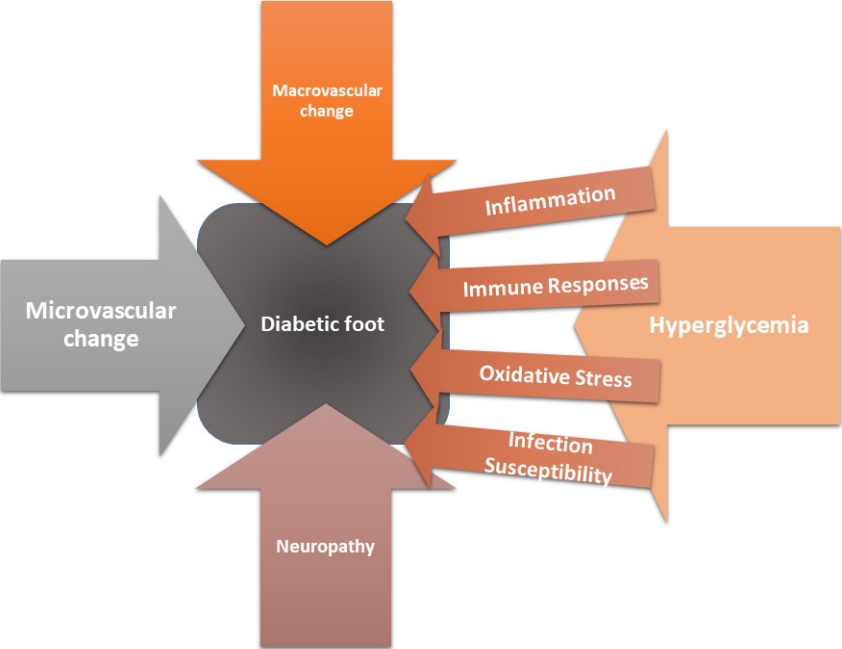
Diabetic foot complications stem from intricate interactions between macrovascular and microvascular changes, neuropathy, inflammation, immune responses, hyperglycemia, oxidative stress, and infection susceptibility. Macrovascular factors like atherosclerosis lead to tissue ischemia, while microvascular dysfunction worsens perfusion deficits. Neuropathy contributes significantly to such complications, causing sensory loss, motor problems, and autonomic dysfunction. This results in unnoticed injuries, muscle atrophy, deformities, and dry skin, elevating the risk of non-healing wounds and infections. Inflammation and immune responses amplify tissue damage and hinder healing. Chronic hyperglycemia generates advanced glycation end products, stiffening tissues, while oxidative stress exacerbates damage. Mitochondrial dysfunction further compromises cellular energy production, worsening tissue repair challenges. These multifaceted factors collectively contribute to diabetic foot complications. The impact of modifiable behavior factors such as smoking, heavy alcohol consumption, and exercise on the risk of lower extremity amputation (LEA) in diabetic patients is also not to be ignored. Substantial data had identified increased LEA risk from smoking and heavy alcohol intake and reduced risk from regular exercise. The cumulative impact of these behaviors underscores the significance of behavior modification in preventing LEA and enhancing the well-being of diabetic patients. Understanding these mechanisms is vital for developing effective preventive strategies, diagnostics, and treatments, addressing the impact of diabetic foot complications on individuals and healthcare systems.
Introduction
Diabetic foot is a complication of diabetes brought on by multiple factors including peripheral neuropathy and vasculopathy due to complex metabolic pathways [1-3], with rising prevalence linked to increased diabetes rates and longer life expectancy [2]. Foot ulcers affect 15% to 25% of diabetic patients [4,5], significantly impacting their lives and mortality rates [6,7]. Remarkably, 28% of these ulcers lead to lower limb amputations [8], with a 5-year mortality rate ranging from 42% to 79% [9,10]. In Korea, the prevalence of diabetes is approximately 14.4% and is especially higher among the elderly, incurring healthcare expenses of 16.2 trillion Korean won (over 12 billion US dollars) a year and approximately 7.7% of total health insurance costs. This study delves into the pathophysiology of diabetic foot and explores ways to prevent amputations through behavioral modifications. Through an extensive literature review, I aim to illuminate this condition’s complexities and propose effective strategies for mitigation and prevention.
Pathophysiology of diabetic foot
Diabetic foot complications arise from complex interplay between macrovascular and microvascular alterations, neuropathic changes, inflammatory processes, immune responses, persistent hyperglycemia, oxidative stress, and an increased susceptibility to infections (Fig. 1).
Macrovascular changes
Macrovascular changes in the diabetic foot are primarily characterized by atherosclerosis, a chronic disease affecting larger blood vessels [11]. The process begins with hyperglycemia, a hallmark of diabetes that triggers a cascade of events that culminate in endothelial dysfunction [12]. Elevated blood glucose levels cause damage to the delicate endothelial cells lining the arterial walls. This damage sets the stage for inflammation, which leads to the deposition of fatty plaques within the arterial walls [13,14]. Over time, these plaques accumulate and harden, narrowing the arteries and reducing blood flow to the lower extremities. The consequences of macrovascular changes are profound. Reduced blood flow to the feet can result in chronic ischemia, depriving tissues of the oxygen and nutrients they need to function properly [15]. This impaired circulation contributes to tissue damage and increases the risk of complications such as ulcers and infections. Moreover, macrovascular changes can also lead to thrombosis within the arteries, further exacerbating the issue by obstructing blood flow.
Microvascular changes
Microvascular changes in the diabetic foot are equally significant, if not more so, than macrovascular changes. These changes predominantly affect small blood vessels including arterioles, capillaries, and venules [12]. One of the hallmark features of microvascular changes is diabetic microangiopathy. Chronic hyperglycemia again plays a pivotal role in initiating and perpetuating these changes. In diabetic microangiopathy, the basement membranes of small blood vessels thicken with an increase in capillary permeability [16]. This results in a loss of normal vascular regulation and function. Oxygen and nutrient delivery to the tissues become compromised, and waste product removal is impaired [17]. The result is tissue hypoxia, which can lead to cell dysfunction and death. The implications of microvascular changes are significant. Reduced blood supply to the foot tissues increases vulnerability to injury and hampers the body’s ability to heal wounds [18]. This diminished perfusion contributes to tissue breakdown, ulcers, and poor wound healing and impairs the normal function of sweat glands and skin, making the skin more prone to dryness and cracking.
Neuropathy
Neuropathy is another critical aspect of diabetic foot pathophysiology. Diabetic neuropathy affects both the somatic and autonomic nervous systems [19]. Sensory neuropathy is perhaps the most well-known form of neuropathy in diabetes and results in loss of protective sensation, making patients less aware of injuries or trauma to their feet [20]. Patients may step on sharp objects, develop blisters, or sustain minor injuries without realizing it [21]. This lack of pain perception can lead to the development of neuropathic ulcers, a hallmark feature of diabetic foot complications. Motor neuropathy, on the other hand, can lead to muscle weakness and deformities in the feet [22]. Muscles may atrophy, and patients can develop altered gait patterns that increase pressure on specific areas of the foot [23]. This abnormal mechanical stress further predisposes the diabetic foot to injury and ulceration. Autonomic neuropathy impacts the autonomic nervous system, which controls functions such as blood pressure, heart rate, and sweat production [20]. In the diabetic foot, autonomic neuropathy can lead to alterations in skin blood vessel tone, causing unpredictable changes in blood flow. This can result in episodes of hyperemia or ischemia, both of which contribute to the risk of foot complications [21].
Hyperglycemia
Hyperglycemia is the defining feature of diabetes and underlies many of the pathophysiological changes observed in the diabetic foot [24]. Elevated blood glucose levels promote oxidative stress and inflammation, disrupt metabolic pathways, and impair cellular function. One of the key mechanisms through which hyperglycemia exerts its damaging effects is the formation of advanced glycation end-products (AGEs) [25]. AGEs are products of non-enzymatic reactions between glucose and proteins or lipids. They accumulate in tissues, where they contribute to inflammation, oxidative stress, and tissue damage. In the diabetic foot, AGEs are increased in number and can promote the development of ulcers and hinder wound healing [26].
Inflammation
Inflammation is a hallmark of diabetic foot pathophysiology. Elevated glucose levels in diabetes activate proinflammatory pathways, leading to the release of cytokines such as tumor necrosis factor-alpha and interleukin-6 [27]. These cytokines promote inflammation and immune cell recruitment in the affected tissues. The chronic inflammatory state observed in the diabetic foot has several detrimental consequences. It compromises the immune system’s ability to mount an effective defense against pathogens. Immune cells may become less responsive, impairing the body’s ability to control infections [28]. Additionally, chronic inflammation contributes to tissue damage and the development of fibrosis, further hindering tissue repair and regeneration.
Immune responses
In diabetes, immune responses are often compromised due to the chronic inflammatory state and impaired immune cell function. Hyperglycemia impairs the function of immune cells such as neutrophils and macrophages, making them less effective at combating infections [29]. Neutrophils, which play a crucial role in fighting bacterial infections, exhibit reduced chemotaxis and impaired phagocytosis in the presence of high blood sugar levels. This impaired neutrophil function increases susceptibility to bacterial infections, which are common in diabetic foot ulcers [30]. Macrophages, important in wound healing and infection control, also experience dysfunction in the hyperglycemic environment [31]. They may exhibit delayed or impaired wound healing responses, contributing to the chronic nature of diabetic foot ulcers.
Oxidative stress
In the context of diabetes, elevated blood glucose levels precipitate oxidative challenges, stemming from an incongruity between the generation of reactive oxygen species (ROS) and the physiological capacity for their detoxification [32]. It is worth noting that ROS are potent entities with the capability to impair cellular structures, encompassing proteins, lipids, and DNA [33]. Oxidative stress plays a multifaceted role in the pathophysiology of the diabetic foot. It contributes to endothelial dysfunction, impairing the function of blood vessels and reducing blood flow [34]. Additionally, oxidative stress can damage peripheral nerves, exacerbating neuropathy [35]. It also damages tissue repair processes, hindering wound healing.
Infection susceptibility
One of the most concerning aspects of the diabetic foot is its heightened susceptibility to infections. The combination of neuropathy, impaired circulation, hyperglycemia, inflammation, and immune dysfunction creates an environment conducive to microbial invasion [36].
The impaired sensation resulting from sensory neuropathy means that patients may not notice small injuries or infections until they have progressed significantly [37]. Even minor cuts or blisters can become entry points for bacteria. Furthermore, the compromised immune responses and reduced blood flow make it difficult for the body to control and resolve infections [38].
Chronic hyperglycemia also provides a favorable environment for microbial growth. Elevated glucose levels can serve as a source of nutrients for pathogens, further promoting infection [39].
Modifiable behavioral factors to prevent LEAs in diabetic patients
A comprehensive study conducted on a Korean population demonstrated that in diabetic individuals, the likelihood of lower extremity amputations (LEAs) escalated with active tobacco use and significant alcohol intake [40]. Conversely, consistent physical activity appeared to mitigate this risk. Modifying current smoking habits, reducing heavy alcohol intake, and incorporating regular exercise can help prevent LEA in diabetic patients (Fig. 2) [40]. Additionally, the risk of LEA increases synergistically with the addition of unhealthy behaviors, with the highest risk observed in individuals who lack exercise, engage in current smoking, and consume heavy amounts of alcohol.
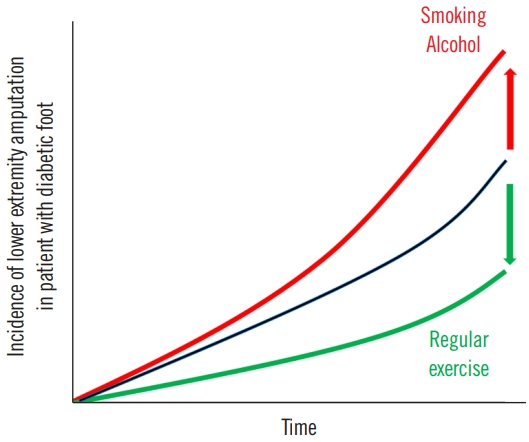
Cumulative incidence of lower extremity amputation in diabetic patients. Demonstrating the role of behavioral factors.
A comprehensive meta-analysis incorporating five cohort studies and three case-control studies highlighted the significant association between cigarette smoking and increased risk of LEA in diabetic patients without publication bias [41]. The pathophysiology of smoking-induced damage lies in the reduced oxygen-carrying capacity of the blood due to harmful cigarette by-products, resulting in tissue hypoxia and arteriospasm [42]. This, in turn, leads to compensatory erythrocytosis, increasing blood viscosity while decreasing tissue perfusion, ultimately inhibiting diabetic ulcer healing and elevating the risk of LEA [43].
Furthermore, sustained and even moderate intake of alcohol in diabetic patients can result in elevated blood glucose levels and peripheral nerve damage. Such conditions can promote diabetic ulcers and heighten the risk of LEA [44]. This highlights the adverse effect of heavy alcohol consumption on LEA risk. Chronic alcohol intake with diabetes often correlates with poor compliance regarding diet and medication, which further hampers glycemic control [45].
On a positive note, evidence from six controlled clinical trials suggests that regular physical activity and exercise can significantly improve diabetic foot outcomes and help prevent complications including diabetic ulcers, infections, and LEA [46]. Exercise in diabetic patients has shown benefits in terms of improved glycemic control, enhanced nerve velocity conduction, and better gait function [47,48]. Furthermore, it delays the onset of diabetic peripheral neuropathy, a pivotal risk factor for diabetic ulcers, by enhancing sensory and motor nerve velocity conduction in the lower limbs [49,50]. Regular exercise also improves balance, foot rollover, dynamic plantar loading, and overall quality of life in diabetic patients [47,50]. Remarkably, weight-bearing from physical activity does not pose an increased risk of diabetic foot re-ulceration [51].
Conclusion
The pathophysiology of the diabetic foot is a complex and multifaceted process that involves a combination of macrovascular and microvascular changes, neuropathy, inflammation, immune responses, hyperglycemia, oxidative stress, and increased susceptibility to infections. These factors interact and amplify each other, creating a hostile environment within the foot tissues. Understanding the intricate interplay of these pathophysiological mechanisms is essential for healthcare providers to effectively prevent, diagnose, and manage diabetic foot complications, ultimately improving the quality of life for individuals living with diabetes. In addition, modification of behaviors of current smoking, heavy alcohol intake, and exercise can help prevent LEA and can improve the physical, emotional, and social qualities of life in diabetic patients.
Notes
No potential conflict of interest relevant to this article was reported.