Management of the Leaky Wound After Arthroplasty: A Review
Article information
Abstract
Total knee and hip arthroplasty are widely performed surgeries for end stage osteoarthritis with highly successful outcomes. However, prolonged postsurgical wound drainage leads to slower healing and predisposes to periprosthetic joint infection. Persistent wound discharge after joint arthroplasty affects patient satisfaction and outcome measures, can cause unexplained re-admissions, and increases the cost burden on the health system. Preventive measures are crucial and include preoperative nutritional assessment, achieving adequate hemostasis, minimizing dead spaces and watertight wound closure. Monitoring these patients with serial C-reactive protein levels is strongly recommended. Many management strategies have been described for such complications, including cessation of deep venous thrombosis prophylaxis, dressings and wound care, antimicrobial therapy, surgical washout and polyethylene insert replacement. However, these conditions are not addressed thoroughly in the literature and optimal management protocol for such complications is still lacking. This article aimed to review the best available literature to date and summarize the findings to help physicians treating these wounds develop objective guidelines in identifying and managing such conditions.
Introduction
Total knee and hip arthroplasty are widely performed surgical procedures for end stage osteoarthritis. However, various early and late complications have been reported with these arthroplasty procedures. Prolonged surgical wound drainage is one of these complications, which can result in unfavorable patient outcomes, delayed wound healing, and periprosthetic joint infection (PJI) [1]. The incidence of prolonged wound leakage after joint arthroplasty is wide and ranges between 0.2% and 21% [2]. However, up to half of the patients with prolonged wound leakage are at risk of developing surgical wound infection [3-5]. Therefore, early and proper management of such complications is crucial. Hip and knee prosthetic infections are extensively discussed in the literature, including their diagnosis, management protocols, and prevention measures. However, the management approach of persistent wound drainage after arthroplasty is sparsely described. This article aimed to review the current evidence in the management of wound leaking after knee and hip arthroplasty.
Methods
We conducted a narrative review of the available studies discussing the topic of persistent wound leakage after hip and knee arthroplasty. Articles were searched in the PubMed database by the two authors, using keywords “wound drainage,” or “wound leak,” and “knee arthroplasty” or “hip arthroplasty.” We included English-written articles of all levels of evidence, regardless of the publication date. The relevant articles that met the inclusion criteria were analyzed regardless of publication date.
Definition
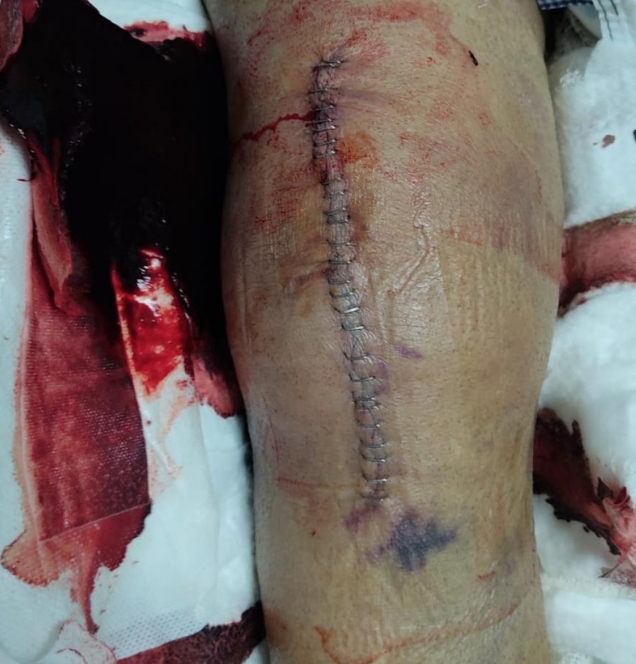
Leaky wounds are defined as those having prolonged drainage (Fig. 1). The first international consensus meeting of musculoskeletal society groups with regard to PJI was held in 2013 [6]; to date, the most objective definition of this condition is described as persistent wound drainage of more than 2×2 cm in dressing gauze 3 days after surgery. These leaky wounds are classified as either limited, moderate, excessive or massive (Table 1) [2].
Serological and radiological investigations
C-reactive protein (CRP) is the most widely used serum marker in patients with delayed wound healing [2]. It is expected to rise in the early postoperative period and return to normal level in 2 to 8 weeks after surgery [2,7]. The acceptable cutoff value of this rise is not clearly defined. Any increase in the level after third day postoperative may suggest PJI [2,8,9]. Biweekly repetition of CRP tests is accepted to see the trends in CRP levels [10]. Wound swab culture has no role, as simple contaminants can cause misleading results and confusion of appropriate management [6,11]. There are no objective guidelines for the role of ultrasonography. It can be used along with clinical judgments, especially in the hip joint, to assess the amount of fluid collection.
Predisposing factors
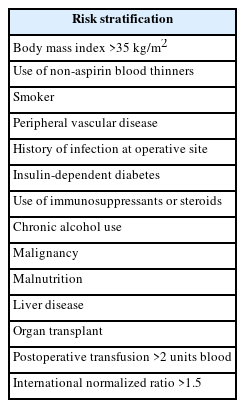
Patients on therapeutic anticoagulation medications are predisposed to develop wound complications [12,13]. Though it is considered safe to perform surgery on patients on aspirin, there are no studies to suggest whether these patients develop these wound-related complications. Patients on disease modifying anti-rheumatic drugs and steroids also have a higher chance of developing persistent wound discharge. Hence, as of now, it is best to be extremely vigilant during the postoperative period of patients on these medications (Table 2) [14,15]. Prophylactic antibiotics are strongly recommended prior to joint arthroplasty. In addition to their rule in reducing the risk of postoperative surgical wound infection, prophylactic antibiotics also lower the incidence of prolonged postoperative wound discharge.
Intraoperative factors
There are patients who have more blood oozing during surgery as compared to others [13]. Suction drains should be considered for these patients. The published literature compares the results of patients with drains inserted and those with no drains [16]. Though drain insertion added no major clinical benefits in a 2018 systematic review, further studies are nevertheless recommended to obtain a more patient and situationspecific conclusion. The use of tranexamic acid has been shown to reduce bleeding and the incidence of postoperative transfusion [17,18]. However, there are no studies to show a reduction in postoperative wound drainage. Based on the available literature, it is highly recommended to use systemic tranexamic acid routinely to infiltrate the wound towards the end of the surgical procedure [19-21]. Watertight wound closure has been described to reduce dead space and therefore the risk of wound drainage [22,23]. Local application of vancomycin powder after arthroplasty has been reported to result in persistent wound discharge [24]. The use of a pneumatic tourniquet during knee arthroplasty surgery has been studied extensively in the literature. Vandenbussche et al. [25] found that tourniquet use minimizes total calculated blood loss, however without a decrease in transfusion rates. Furthermore, the tourniquet reduces oxygenation to the wound edges and increases postoperative wound complications [13,26,27]. Inadequate oxygen delivery to soft tissue inhibits the shifting of inflammatory cells into the wound and therefore can compromise soft tissue repair [28]. Recent evidence revealed a strong correlation between postoperative wound leakage and prolonged tourniquet application [13]. Currently, there is no solid recommendation for using suction drains in primary uncomplicated knee arthroplasty. However, a larger amount of drainage increases the risk of wound leaking in both hip and knee arthroplasty [29].
Effect of postoperative chemical deep vein thrombosis prophylaxis
Low molecular weight heparin (LMWH) is commonly used to prevent the development of deep vein thrombosis following arthroplasty. However, there have been reports of prolonged wound drainage associated with its use [30,31]. Some LMWH agents are so potent in their action they cause leaky wounds, for which the term “Clexane knee” was coined. The latest American Academy of Orthopedic Surgeons and National Institute for Health and Care Excellence guidelines have accepted aspirin as acceptable prophylaxis except for high-risk cases [32,33]. If a patient develops prolonged wound drainage after surgery LMWH must be stopped, and aspirin and/or mechanical prophylaxis such as pneumatic compression devices and compression stockings should be considered along with ice and rest for the operated part.
Negative pressure wound therapy
Negative pressure wound therapy (NPWT) is a relatively newer modality of treatment in which a special dressing is used to apply subatmospheric pressure continuously or intermittently [34]. Traditional NPWT draws fluid into a canister but singleuse NPWT (sNPWT) applies negative pressure to the wound through an integrated battery powered pump [35]. This sNPWT can also be used for discharging wounds. The international consensus meeting has recommended occlusive dressings for all arthroplasty cases with moderate consensus [11]. However, there was a strong consensus on the use of sNPWT for complex cases [16,36]. Table 3 summarizes the prophylactic indications for NPWT in arthroplasty cases.
Evidence-based approach to patients with leaky wounds
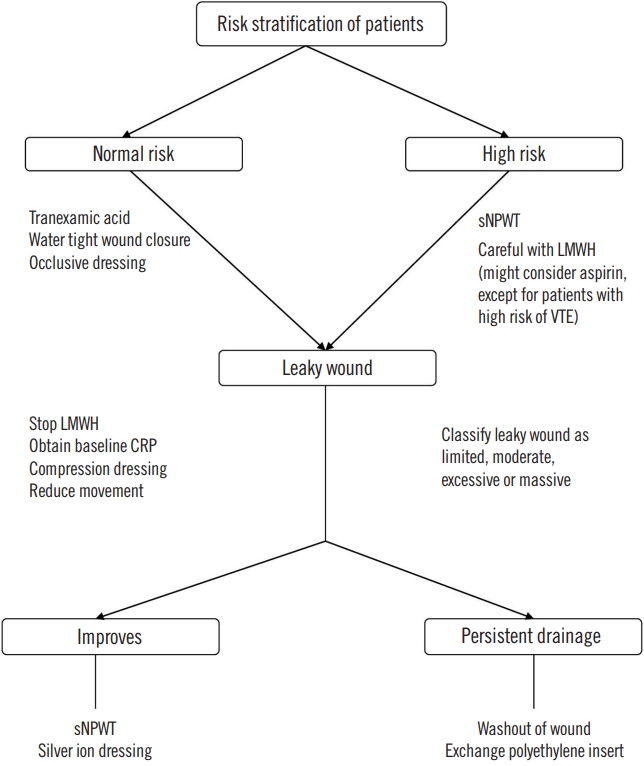
A patient with persistent wound drainage 4 days after the index surgery should be evaluated and classified. Limited grade leaky wounds can initially be managed conservatively. This involves stopping LMWH administration and changing to aspirin and/or mechanical prophylaxis. A baseline CRP level should be obtained. Wound swab cultures have no role as wound swabs are often contaminated. Ice application along with reducing movement of the affected joint is helpful [2]. Knee flexion of more than 30° is considered as the best position [37]. NPWT dressings can be applied as well [36,38]. The addition of silver dressings has been shown in the literature to further reduce the chances of infection [39]. In addition to their antibacterial role, silver dressings reduce the amount of wound drainage by minimizing dead space and therefore enhancing wound healing [40,41]. Guirro et al. [42] recommend extended use of antibiotics to avoid the development of deep infection although experts in the field are advising against this practice.
Fig. 2 summarizes the authors’ approach to patients with post-knee arthroplasty leaky wounds. Cases presenting with persistent discharge after 5 to 7 days have to be managed in the operative theater for joint washout and change of modular components [43−46]. Adequate hemostasis needs to be achieved in case of any major bleeding. These cases should be managed as early PJI. Watertight closure with barbed sutures should be performed and monofilament sutures should be used for skin closure. Staples have been associated with a higher incidence of persistent wound drainage [47]. The nutritional status of the patient should be assessed by measuring serum albumin and transferrin levels. Replacement therapy should be initiated in consultation with nutritionists. In rare cases, if the discharge persists after the initial washout the patient should be considered to have deep PJI and staged reconstruction should be considered.
Conclusion
Early and proper management of wound leakage after arthroplasty is essential to prevent deep joint infection. Persistent drainage for more than 5 to 7 days or cases not responding to primary measures will necessitate wound exploration, washout, and change of polyethylene inserts.
Notes
No potential conflict of interest relevant to this article was reported.