Undiagnosed Behçet Syndrome Presenting with Abdominal Wall Ulceration: A Case Report
Article information
Abstract
Behçet syndrome (BS) is a chronic inflammatory disease that damages blood vessels and affects multiple systems, including the skin, mucosa, joints, eyes, nervous system, and gastrointestinal tract. The disease follows a relapsing course, characterized by recurrent episodes of inflammation. Cutaneous lesions are a common manifestation of BS, presenting as ulcerations exhibiting the pathergy response, posing a challenge for healing. We report an unusual case of abdominal wall ulceration in a female patient with previously undiagnosed BS. The atypical characteristics and course of the ulcerations, as well as the data obtained from the patient’s medical history and laboratory findings, prompted a multidisciplinary approach. The findings, in conjunction with the clinical suspicion, resulted in a diagnosis of BS. Following the diagnosis, the patient received appropriate management, including local wound care and medical treatment for BS, resulting in rapid wound epithelialization within a few days of treatment initiation. The patient was referred to a rheumatologist for further evaluation and long-term management of BS. This case underscores the significance of including BS in the differential diagnosis of unusual ulcerations.
Introduction
Behçet syndrome (BS) is a syndrome of unknown etiology that is characterized by a relapsing and remitting course [1,2]. Patients may present with diverse clinical features that occasionally overlap, involving the skin, mucosa, joints, eyes, vessels, and nervous and gastrointestinal systems [3]. The hallmark of BS is mucocutaneous lesions, with oral and genital ulcers, papulopustular and nodular lesions being the most common manifestations [4]. Although unusual abdominal wall ulcerations related to BS are rare, they can present in an atypical manner, posing a diagnostic and therapeutic challenge. This case report describes an unusual abdominal wall ulceration related to undiagnosed BS that persisted for an extended period. The report was approved by the Institutional Review Board of Soonchunhyang University Hospital (IRB No. 2023-03-007). The patient provided written informed consent for the publication and use of her images.
Case
A 56-year-old woman with no significant medical history underwent robotic total hysterectomy and bilateral salpingo-oophorectomy for pyometra with fever and vaginal discharge. Though the patient was discharged without complications, she re-visited the hospital 3 months after surgery because of a suspected delayed surgical site infection, including pus-like discharge, pain, and induration at the laparoscopic site. The patient then received empirical third-generation cephalosporin antibiotics and underwent contrast-enhanced abdominopelvic computed tomography to evaluate the extent of the infection and find any intra-abdominal abnormalities (Fig. 1). The patient also underwent debridement of devitalized tissues several times in the operating room (Fig. 2), and microbiological Gram and Ziehl-Neelsen stains, culture, and polymerase chain reaction tests were performed on pus and deep tissues for bacteria, Mycobacterium tuberculosis, nontuberculous mycobacteria, and fungi.
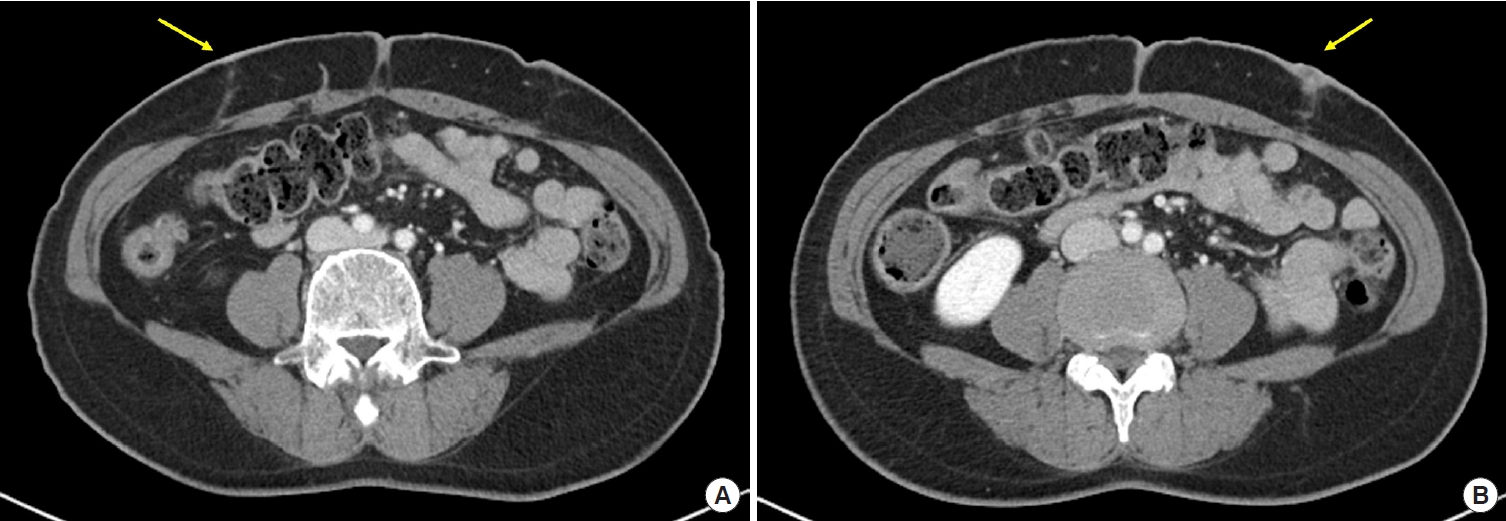
Abdominopelvic computed tomography images. Demonstrating a lesion with peripheral contrast enhancement, which is suspected to be a complication of a skin wound on the abdominal wall (yellow arrow). No evidence of associated abscess or intra-abdominal communication was found. (A) The right aspect of the abdomen. (B) The left aspect of the abdomen.

Intraoperative photographs. Demonstrating a meticulously debrided wound bed that is free of necrotic tissue following the initial surgical intervention. (A) The right aspect of the abdomen. (B) The left aspect of the abdomen.
Blood tests with a complete blood count, chemistry, and inflammatory markers displayed normal results, and the microbiological tests were all negative. Abdominopelvic computed tomography confirmed a shallow ulceration without intra-abdominal abnormalities and communication. As there was no evidence of bacterial infection, revision surgery was performed after debridement. However, despite topical wound healing with dressings and multiple debridements repeated for 2 weeks, the wound continued to progressively widen and deteriorate (Fig. 3).
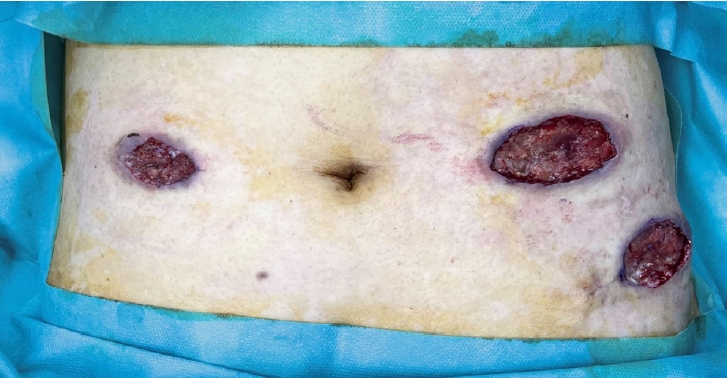
Photograph of the patient’s abdomen. Despite 2 weeks of conventional wound care, the wound exhibits non-epithelialization, absence of peripheral contracture, and increased size.
A multidisciplinary approach was taken to investigate the atypical clinical course, and the patient was diagnosed with BS based on positive human leukocyte antigen B51 and lupus anticoagulant tests, as well as a positive pathergy test. The patient was transferred to the department of rheumatology and started on immunosuppressive therapy, including steroids, mycophenolate mofetil, cyclosporine, intravenous immunoglobulin, and infliximab targeting BS.
Within a week of treatment, the wounds were epithelialized, and the symptoms of pain and pus resolved (Fig. 4). The patient has been on long-term immunosuppressant treatment, including steroids and infliximab, for BS and the wound has remained stable during a 2-year follow-up (Fig. 5).

Photograph of the patient’s abdomen. Exhibiting rapid epithelialization within 1 week of commencing systemic treatment for Behçet syndrome.
Discussion
BS is a chronic recurrent systemic vasculitis that mainly affects small vessels, causing recurrent and chronic symptoms across various organs. Diagnosis of BS is challenging since no laboratory test is definitive; therefore, clinical evaluation is essential [5]. To be diagnosed with BS, a patient needs to have a score of 4 or more based on the following point system: oral ulcers, genital ulcers, and ocular lesions are each worth 2 points, while skin lesions, vascular manifestations, and neurological manifestations are each worth 1 point. Hence, if a patient has any of these symptoms, BS should be suspected [5]. Diagnosing our patient was challenging due to the absence of the hallmark symptoms of BS.
Although rare, ulcerations related to BS require specialized wound care management that integrates systemic therapy [6]. Due to the clinical heterogeneity of BS and its unpredictable course, choosing the appropriate systemic therapy during active disease is based on the most prominent disease manifestation, such as arthritis, gastrointestinal disease, mucocutaneous manifestations, neurologic disease, ocular, or vascular disease (Table 1). Additionally, adherence to the following principles of wound care is crucial to promote optimal healing outcomes [7,8].
First, if BS is suspected, it is best to avoid performing sharp debridement, because it can lead to an exaggerated inflammatory response that may cause the wound to remain “stuck in the inflammatory phase of healing,” thereby delaying the healing process [9]. In cases where debridement is necessary due to the presence of excessive necrotic or fibrotic tissue in the wound bed, other debridement methods such as mechanical debridement, enzymatic debridement, or biologic debridement should be considered [8,10].
Second, topical corticosteroids, such as 0.1% triamcinolone acetonide cream, possess potent anti-inflammatory properties and can be effectively used to induce remission in BS ulcers. Application of the cream should be done three to four times daily until the pain from the ulcer subsides. Topical sucralfate (1 g/5 mL) can be used in conjunction with or as an alternative to topical corticosteroids. In some cases, triamcinolone (40 mg/mL) can be injected into the ulcer edge to promote healing, combined with systemic treatment [11,12].
Lastly, delayed wound healing after surgery can be associated with various factors, including underlying conditions such as BS [13,14]. In our case, BS was an unexpected diagnosis that was discovered due to the patient’s atypical wound healing process and delayed surgical site complications. Therefore, in addition to appropriate local wound care, clinicians should consider BS as a potential cause of atypical wound healing and recognize the importance of a multidisciplinary approach to its diagnosis and management. Managing the underlying disease is also crucial in both controlling disease activity and preventing further ulcerations.
In this case report, we describe a female patient with progressive and unusual abdominal wall ulceration related to BS, which was refractory to conventional wound management. Accurate diagnosis of BS enabled the implementation of effective medical and wound care interventions, which led to the successful resolution of the abdominal wall ulcerations and improvement of the systemic disease. This case highlights the importance of considering BS in the differential diagnosis when managing unusual ulcerations and atypical courses of wounds. Early identification and appropriate treatment of the underlying conditions can lead to prompt wound resolution, potentially reducing patient morbidity.
Notes
This work was supported in part by the Soonchunhyang University Research Fund (Grant No. 2023-0036). No other potential conflicts of interest relevant to this article were reported.


