Grafting Low-Temperature Micronized Adipose Tissue Niche to Treat Diabetic Foot Ulcers: A Pilot Study
Article information
Abstract
Background
In a previous study, we suggested that micronized adipose tissue (MAT) niche could be an effective and safe treatment for diabetic foot ulcers. Fibrin glue was used to solidify the MAT niches. However, it took 40 to 50 minutes for the MAT niche to be ready for application. We have devised an alternate method in which the MAT niche is created at a low temperature to reduce the solidification time. This pilot study aimed to investigate the possibility and safety of grafting a low-temperature MAT niche for treating diabetic foot ulcers.
Methods
Patients with diabetic foot ulcers who were treated with low-temperature MAT or conventional methods between March 2020 and December 2021 were included in this study. We evaluated the efficacy of the treatment by measuring the wound size reduction rate. All adverse events were recorded.
Results
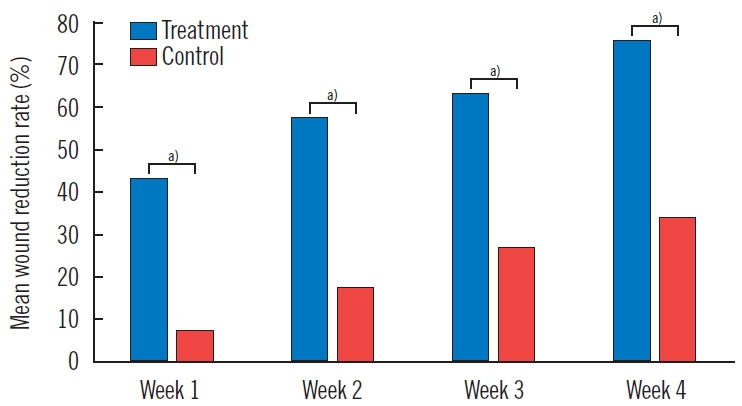
The MAT niche treatment group showed a statistically significant wound reduction rate during the entire study period of 4 weeks (P<0.005). The wound reduction rates in the 4th week after the treatment were 75.7%±16.9% in the treatment group and 33.5%±19.0% in the control group (P=0.001). No serious adverse events related to low-temperature MAT niche treatment were observed.
Conclusion
This pilot study demonstrated the potential of low-temperature MAT niche as an effective and safe method for the treatment of diabetic foot ulcers.
Introduction
It is a broadly accepted consensus that patients with diabetes exhibit impaired wound healing at the molecular level because of factors including inadequate blood supply, infection, inhibited wound healing material migration, and lack of growth factors [1-3]. Our Diabetic Wound Center has focused on several autologous or allogenic cell-based therapies to treat diabetic wounds. Grafting uncultured stromal vascular fraction (SVF) cells, derived from adipose tissue, has shown favorable results. Moreover, adipose tissue is relatively easy and safe to harvest. However, despite their reliability, SVF cells still have a few drawbacks. For example, collagenase, which is an essential material for producing SVF, needs the approval of each local Food and Drug Administration before being able to be used in many countries.
To overcome these drawbacks, we hypothesized that micronized adipose tissue (MAT) grafting might be able to replace SVF cell grafting. In a previous pilot study, we demonstrated the possibility of MAT grafting in treating diabetic foot ulcers. This was the first clinical study because the effects of MAT grafting on wound healing had been previously reported only in in vitro and in vivo studies [4]. We named this procedure “MAT niche grafting” in order to emphasize its 3-dimensional (3D) printed nature, which effectively replaces wound defect volume. In this previous pilot study, fibrin glue was used to solidify the MAT niche. However, it took 40 to 50 minutes for the MAT niche to be ready for application in diabetic wounds.
As an alternative, we have devised a method to create the MAT niche at a low temperature to reduce the solidification time. This pilot study was designed to assess the possibility of using a low-temperature MAT niche for treating diabetic foot ulcers.
Methods
The low-temperature MAT niche grafting method was approved by the Institutional Review Board of Korea University Guro Hospital, as was this retrospective study (no. 2020GR0590 and no. 2015GR0181, respectively). This study was conducted in accordance with the Declaration of Helsinki and informed consent was obtained from each patient.
Patients
Patients who were treated at our Diabetic Wound Center between March 2020 and December 2021 and met the following inclusion criteria were included in this retrospective study. The inclusion criteria were: age more than 19 years, diagnosed diabetes duration longer than 5 years, presence of lower extremity ulcers, transcutaneous oxygen pressure >30 mmHg, weekly visits to our center with serial clinical photos taken, and treatment by low-temperature MAT niche or conventional management. Patients who exhibited clinical signs, magnetic resonance imaging findings, or positive microbiological culture results indicating infection such as cellulitis or osteomyelitis were ineligible for participation in the study. Patients were also excluded if they were diagnosed with diseases that could interact with wound healing (e.g., malignant tumors, immunosuppressed state, systemic infection state, or connective tissue disorders).
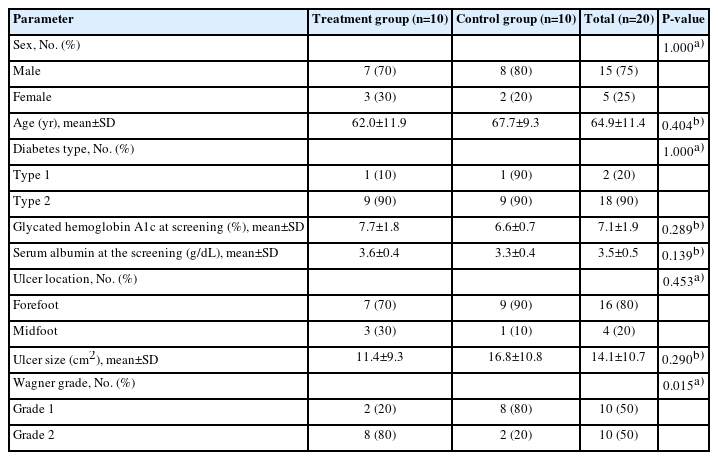
Overall, 20 patients with diabetic foot ulcers, comprising 15 men and five women with a mean age of 64.9±11.4 years (range, 33–82 years), were included in this study. Among the 20 patients, 10 were treated with low-temperature MAT niche graft (treatment group) and 10 with standard wound care (control group). Baseline patient demographics for the two groups are shown in Table 1. No statically significant differences were observed in any clinical characteristics between the two groups except the Wagner grade.
Treatment group: low-temperature MAT niche
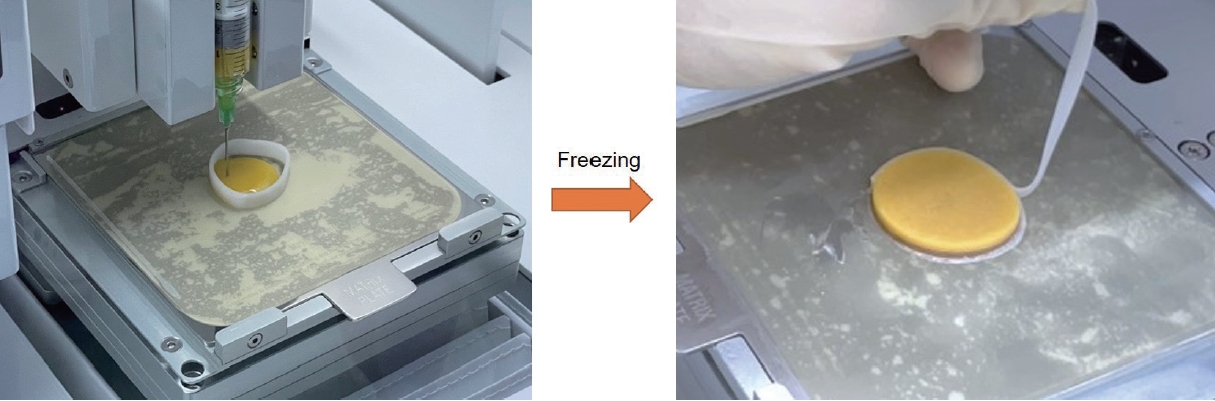
To harvest autologous adipose tissue, liposuction was gently performed on the patient’s abdominal area under local anesthesia by a board-certified plastic surgeon. To reduce the particle size of adipose tissue with minimal manipulation, a large-pore micro-mesh disc filter was firstly used to discard cellulose and/or debris from the freshly obtained fat. Other sterilized micronizers with different pore sizes (4,000, 2,400, 600, and 200 μm) were used to harvest extracellular matrix (ECM) from adipose tissue. After filtering and micronizing, sterile saline was added in a 1:2 ratio and the resulting compound was transferred into syringes which were inverted to mix the contents. As the next step, the syringes were placed vertically in a rack for 10 minutes to let gravity separate the mixture into a top layer of free oil, a middle adipose layer and a bottom layer of saline, blood and debris. The saline layer was discarded to obtain a solution of concentrated adipose-derived ECM, which was then transferred to new syringes for use as a bio-ink, to be dispensed through a 3D bioprinter (Dr. INVIVO, ROKIT Healthcare).
To create a 3D patch, the syringes containing the patient’s adipose tissue-derived bioink were loaded into the 3D bioprinter. The bioprinter first printed a scaffold matching the wound dimensions from Dispenser 1 using poly-caprolactone (PCL), after which Dispenser 2 dispensed the bio-ink into the scaffold to complete the customized patch. All of the printing operations were conducted in a temperature-controlled room maintained at 22±2 °C, which took more or less 10 minutes to complete, depending on the patch’s size and complexity. The resulting patch consisted of the autologous adipose tissue bio-ink forming the interior and PCL making up the exterior. The print bed, along with the patch, was cooled to an average –15 °C using the built-in bed cooling system of the bioprinter, which froze the patch within 5 to 10 minutes. After about 10 min, the frozen patch was picked up using forceps and applied to the wound (Fig. 1). Lastly, the wound was covered with a sterile silicone wound dressing (Mepitel, Mölnlycke Health Care). The patients were monitored with weekly dressing changes.
Control group: conventional treatment
Daily wound dressings were performed after wound cleansing. The wound was treated with saline-soaked dressings, betadine-soaked dressings, or hydrogel with povidone iodine depending on the wound condition. Patient follow-up was done on a weekly basis with dressing changes and clinical photos taken at every visit.
Evaluation
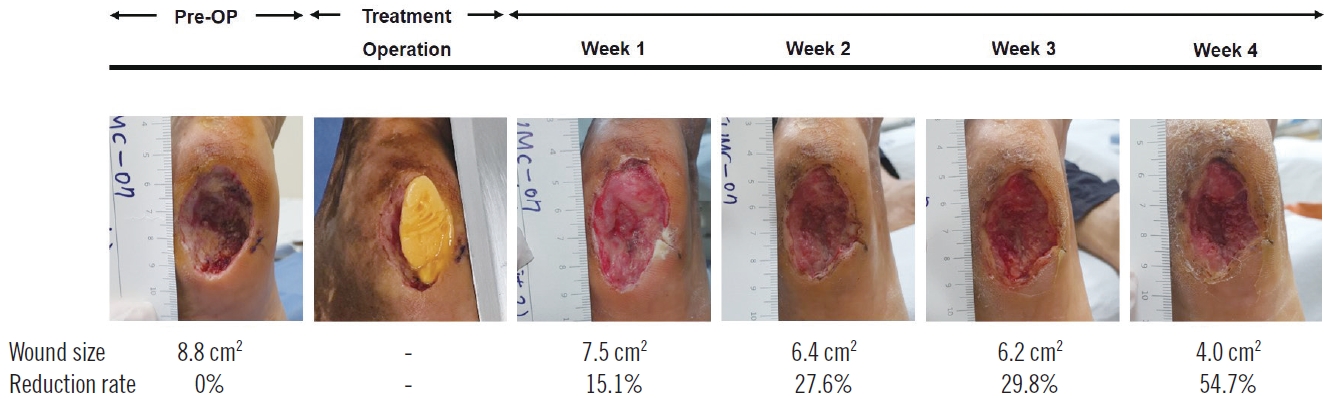
During the study, the patients were evaluated weekly from baseline to the fourth week after treatment. The patients were clinically evaluated and the wound area was measured. Digital photometry of the weekly photos was employed to determine the wound size and the rate of reduction. Safety was assessed by evaluating side effect reports, laboratory assessments, and vital signs throughout the study. Data regarding adverse events in response to treatment were collected from all patients to determine the safety of the treatment (Fig. 2).
Statistical analysis
Fisher exact test was used to compare categorical variables, and the Mann-Whitney test was used to compare quantitative variables. In the treatment group, differences in wound size between every measurement were assessed using the Wilcoxon signed-rank test. Data are expressed as mean±standard deviation. All statistical analyses were performed using SPSS version 20.0 for Windows (SPSS Inc.).
Results
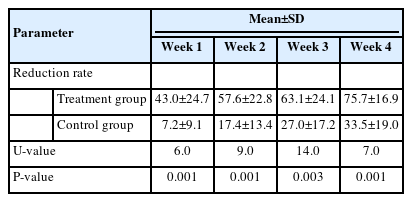
At post-treatment week 4, the wound size was reduced by 7.8±4.9 cm2 (mean±SD) in the treatment group and 5.1±4.1 cm2 in the control group (P=0.199, U-value=33.0) (Table 2, Fig. 3). The treatment group showed a statistically significant wound size reduction between every measurement (P<0.005, Wilcoxon signed-rank test) (Fig. 4). Also, the treatment group showed a statistically significant wound reduction rate among all measurements (P<0.005) (Tables 2 and 3, Fig. 5). The total reduction rates at final follow-up were 75.7%±16.9% and 33.5%±19.0% in the treatment and control groups, respectively (P=0.001) (Tables 2 and 3, Fig. 5). The reduction rate showed a statistically significant difference between the two groups every week (P<0.005) (Table 3, Fig. 5). No complications such as infection, bleeding, or allergic reactions were observed during application of the respective treatments.
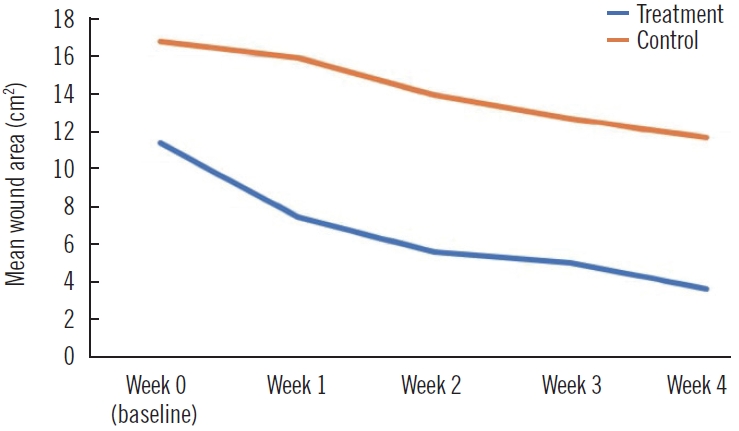
Comparison of mean wound area per week. The wound size was reduced by 7.8±4.9 cm2 in the treatment group and 5.1±4.1 cm2 in the control group (P=0.199, Mann-Whitney U test).

Wound size of treatment group. The treatment group showed a statistically significant wound reduction rate between every weekly wound measurement until the end of the study at week 4. a)P<0.005, Wilcoxon rank sum test.
Discussion
The treatment group showed a statistically significant wound reduction rate at all visits (Tables 2 and 3, Fig. 5). The rate of wound reduction during the 4-week treatment period was significantly higher in patients who received a low-temperature MAT niche compared to the control group (75.7% vs. 33.5%, P=0.001). There were no statistically significant differences in demographics and baseline wound characteristics except the Wagner grade in the two groups. The treatment group showed a significantly higher Wagner grade (P=0.015) (Table 1). The Wagner grade is a well-known tool for evaluating the severity of wounds, especially in diabetic patients [5]. These results indicate that the treatment group demonstrated a higher wound reduction rate regardless of the poorer wound status at baseline. Since the reduction rate of wound size over the course of 4 weeks of a given treatment is widely accepted as a clinical predictor for healing [6,7], these results comprehensively suggest that applying a low-temperature MAT niche may have the potential as an effective procedure for patients with diabetic ulcers.
Diabetic wounds are well-known as one of the most complicated wounds to treat [8]. Our center has conducted fairly extensive studies over 20 years to improve the results of wound care. These patients have a higher risk of infection and usually require serial surgical debridement. Additionally, the re-stenosis or re-occlusion rates are about 50% in patients with a history of lower extremity recanalization for peripheral artery disease; this means that tissue oxygenation could again fall to inadequate levels before wound improvement, contributing as a pathogenic factor to delayed wound healing [9]. For these reasons, wound care specialists need to find an effective method to reduce the wound healing time.
To overcome the various obstacles that diabetes can cause at the molecular level in the wound healing process, researchers have adopted various strategies for treating diabetic ulcers [10-13]. The beneficial effects of MAT grafting on wound healing have recently been reported in in vitro and in vivo studies. However, before our previous pilot study, there were no clinical studies that had documented the effects of MAT on wound healing among patients diagnosed with diabetes. Our previous study suggested the potential of a MAT niche as a simple and favorable treatment for diabetic ulcers with a 77% wound reduction rate within 4 weeks. Complete wound healing was achieved after 16 weeks in eight of 10 patients (80%) in the MAT niche-treated group [4]. These promising results could be explained by the abundance of fundamental wound healing factors in MAT. The role of SVF cells in promoting tissue regeneration and remodeling has been extensively validated through numerous previous studies. SVF cells are the main cellular components of MAT, including fibroblasts, endothelial cells, and adipose-derived stem cells (ASCs). In addition, MAT is composed of ECM and cytokines, which are essential to form a scaffold for healthy tissue regeneration. In the case of patients with diabetes, the wound healing process is impaired at the molecular level. Exposure to high glucose is associated with decreased cell proliferation and migration, poor growth factor production, and abnormal ECM formation [3]. Considering the components and regenerative potential of MAT, MAT niche grafting may be a promising alternative for diabetic foot ulcers. However, the preparation of the MAT niche for diabetic wounds generally took over 50 minutes in our previous study, among which the longest time was spent solidifying the MAT niche. Fibrin glue was originally used as a solidifying material to stabilize the MAT niche. By adding fibrin glue to the MAT niche, it could optimally be applied and grafted to irregular shaped 3D wounds [14,15]. Other solidifying materials may solidify the MAT niche faster, however, the effect of applying such alternative solidifying materials on wounds could be unpredictable, perhaps even hindering the wound healing process in diabetic patients with impaired immune systems.
Therefore, we attempted to solidify the MAT niche at a low temperature as an alternative method. Low temperatures can affect several factors in MAT by modulating its molecular structure. Chronic wound beds deficient in oxygen and nutrients present a harsh environment full of obstacles for grafted frozen cells to survive, proliferate, and heal the wound [16,17]. In 2014, Choudhery et al. [18] reported that adipose tissue could be successfully cryopreserved for future clinical application and that the thawed ASCs, an important component of MAT, were equivalent in functionality to freshly processed ASCs [18]. Several other studies also demonstrated successful cryopreservation of ASCs from fresh adipose tissue, retaining proliferation and differentiation potency [19-21]. In our study, the wound reduction rate in patients with a low-temperature MAT niche applied was significantly higher than in the control group, which shows that this treatment might be an effective procedure for patients with diabetic ulcers. According to our results, the MAT niche may maintain its potential to promote wound healing at low temperatures.
The treatment group did not report any complications, such as infection, hemorrhage, or allergic reactions. The only invasive technique we used in this study was liposuction. Liposuction has been utilized in the field of plastic surgery for several decades, with only minimal occurrence of major complications. The absence of major complications in this study confirms the safety of the low-temperature MAT niche.
This study has several limitations. First, the study included only a small cohort of patients. Further studies with larger cohorts are necessary to confirm the superiority of the MAT autograft over conventional treatment. Second, we did not compare the effects of the lower-temperature MAT niche to those of the MAT niche with fibrin glue. For these reasons, we are planning future studies to compare low-temperature MAT with conventional fibrin glue-solidified MAT with longer follow-up periods.
Notes
This study was partially supported by grants from ROKIT Healthcare, Seoul, Republic of Korea. Except for that, no potential conflict of interest relevant to this article was reported.