Necessity of Tissue Culture from Clinically Uninfected Wounds in Diabetic Patients
Article information
Abstract
Background
The Infectious Diseases Society of America Clinical Practice Guidelines recommends not culturing specimens from patients with diabetes who do not show clinical signs or symptoms of local inflammation. However, in diabetic patients the local or systemic inflammatory response is commonly reduced, even in severely infected cases, owing to poor immune response. This study aimed to investigate the clinical significance of tissue cultures in patients with diabetic ulcers without clinical signs of infection.
Methods
This study included 248 of 1,346 patients who had diabetic foot ulcers but did not exhibit any signs or symptoms of infection in our hospital. Tissue culture results were classified as either negative, positive culture in soft tissue or positive culture in bone. Wound healing outcomes were divided into two categories: healed without amputation or healed with amputation.
Results
A significant difference existed between the negative culture and bone culture-positive groups. Patients who had a positive bone culture result were 2.1 times more likely to require amputation than those who had negative culture results. Moreover, the rate of amputation was higher in bone culture-positive patients than in soft tissue culture-positive patients.
Conclusion
Our study showed that tissue culture may be required for patients with diabetic ulcers even if they do not exhibit any signs or symptoms of inflammation.
Introduction
Diabetic foot ulcers are a common complication of diabetes mellitus but can still develop into life-threatening conditions. Even with proper management, diabetic foot infections (DFIs) can be difficult to control, and amputation may be necessary [1]; infection is a well-known and significant cause of lower-limb amputation [2]. Therefore, accurately identifying infection of diabetic foot ulcers is critical. The prevailing opinion is that identification of DFI should be based on clinical observations rather than microbiological reports of tissue cultures [3-5]. The International Working Group on the Diabetic Foot and Infectious Diseases Society of America (IDSA) has established clinical criteria for DFI, which include the presence of at least two inflammatory symptoms, such as erythema, pain, tenderness, and warmth [6,7]. They suggest that if a wound does not show clinical signs of infection, collecting specimens for culture is not required. The guidelines also suggest that mild infections may not require culture tests.
However, diagnosing infections in patients with diabetes based solely on clinical observations may be challenging. In patients with diabetes, the immune-leukocyte system response is often weak, which can reduce local inflammatory reactions and classical signs or symptoms of local infection [8-10]. Diabetic patients may exhibit less apparent or reduced clinical signs and symptoms of foot infections. They may also not show systemic signs, such as fever, leukocytosis, general malaise, numbness, anorexia, or nausea, even in severe cases of infection, or these signs may be delayed [11-13]. Despite the absence of clinical signs of infection, diabetic wounds can have a high bacterial load, which can lead to subclinical infection or critical colonization. This can negatively affect wound healing.
Depending on the clinical signs required for diagnosis, some infections may not be detected early. If the start of optimal treatment is delayed, the infection may progress. This progress is often rapid owing to diabetic immunopathy, ischemia, and anatomic characteristics of the foot [5]. Therefore, the absence of local and systemic signs or symptoms of inflammation cannot be relied upon for DFI identification. Based on our experience, some diabetic wounds may have a poor prognosis even in the absence of clinical signs or symptoms of infection and with satisfactory tissue perfusion. Thus, the diagnosis of DFI can be controversial. This study aimed to investigate whether tissue culture is necessary for patients with diabetic wounds who do not exhibit clear clinical signs or symptoms of infection.
Methods
The Institutional Review Board of the Korea University Guro Hospital approved the study protocol (#KUGH 15092-001). This study was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all patients.
Management protocol in brief
Basically, all patients were treated according to the International Consensus on the Diabetic Foot guidelines. Complete medical histories were obtained from all patients. Various general serological tests were performed, including those that measure serum blood glucose levels and inflammatory markers. The vascularity of the diabetic foot was evaluated via measuring the transcutaneous partial oxygen pressure (TcPO2) and toe pressure, and examining Doppler waves of the major arteries to the foot. Patients with peripheral vascular disease (PVD) were treated by interventional cardiologists who performed percutaneous transluminal angioplasty. Hyperbaric and normobaric oxygen therapy were used to manage various clinical conditions associated with tissue hypoxia. Instead of swab cultures, a specimen was collected from deep tissue for culture, even if the foot displayed no clear signs or symptoms of infection before starting antibiotic treatment. If necessary, patients were treated with systemic antibiotics empirically, and modifications were made in accordance with the outcomes of culture sensitivity tests. Continuous removal of unhealthy tissue from the wound was performed depending on the wound. Patients diagnosed with osteomyelitis using magnetic resonance imaging (MRI) and bone biopsy culture were administered antibiotics for at least 3 to 6 weeks. If a diabetic wound persisted for more than 4 weeks, MRI was performed on the assumption that osteomyelitis might be present [6]. Neuropathy was assessed using Semmes–Weinstein monofilament, pin-prick, temperature, nerve conduction velocity, and electromyography tests. Depending on the location of the wound, appropriate off-loading was emphasized.
Patients
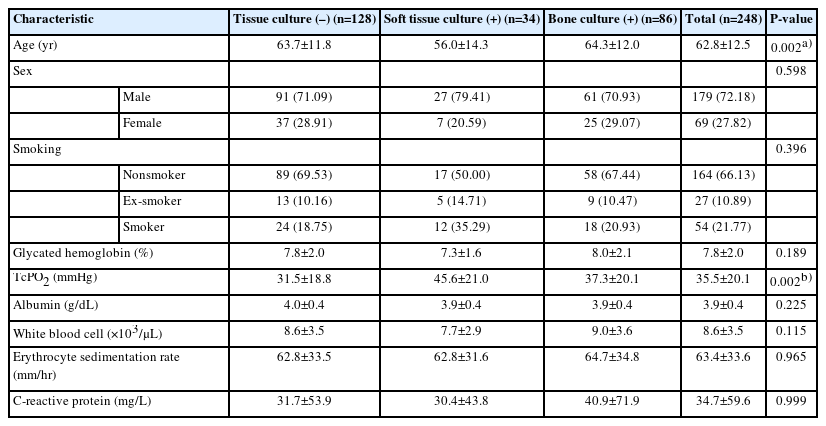
In this study, the medical documents of patients with diabetic foot ulcers who were admitted to the hospital center from January 2008 to December 2021 were examined, with a total of 1,346 documents reviewed. The patient information was linked to the electronic records of the hospital. Linked data were obtained from the Admitted Data Collection in the hospital where the authors conducted their study. Regular audits were conducted to minimize inaccuracies. The patients were managed by a specialized diabetic wound team. The study included patients from a group of 1,346 individuals with diabetic foot ulcers who met certain inclusion criteria: (1) absence of clinical signs or symptoms of infection; (2) accessible tissue culture reports; and (3) accurate documentation regarding the final clinical outcomes of completely healed patients. Ultimately, 248 patients were included in this study: 179 male and 69 female patients with an average age of 62.8±12.5 years.
Determination of infection
The severity of infection was determined through examining the results of tissue biopsy cultures, in which a sample of deep tissue and/or bone was collected after cleaning and removing dead tissue from the wound. Tissue biopsy cultures were used to grade the degree of infection, which was categorized into three groups: I0, I1, and I2. I0 indicated absence of microbial growth in the culture, whereas I1 and I2 indicated positive cultures from soft tissue and bone, respectively. Table 1 shows the patient demographics and ulcer characteristics.
Clinical outcomes
The wound-healing outcomes were classified into two categories: complete healing with or without the need for amputation. The term “healing without amputation” was defined as the complete closure of a diabetic ulcer without the need for surgical intervention involving the removal of any part of the foot. Amputation was classified as partial or complete resection of one or more toes, removal of the proximal foot, below-knee amputation, or above-knee amputation.
Statistical analyses
The Kruskal-Wallis, analysis of variance, chi-square, and Fisher exact tests were used to compare healing outcomes among the three groups (I0, I1, and I2). The chi-square test, Fisher exact test, and odds ratios (OR) with 95% confidence intervals (CI) were used to analyze the association between culture results and healing outcomes. Statistical significance was set at P<0.05. Data were analyzed using SAS version 9.4 (SAS 9.4, SAS Institute Inc.).
Results
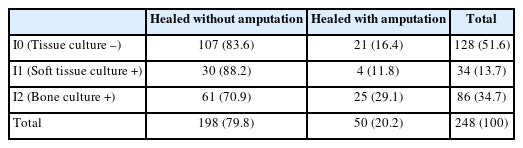
Although no clinical signs or symptoms of inflammation were observed, 120 wounds (49%) showed positive culture results. In 34 (13.7%) patients, bacterial growth was restricted to the soft tissue (I1) and in 86 (34.7%), it extended to the bone (I2) (Table 2).
When analyzing the demographic and clinical characteristics of the study population using statistical tests, such as Kruskal-Wallis, analysis of variance, chi-square, and Fisher exact tests, no significant differences were found among the three groups except for age and tissue perfusion values (Table 1).
In this study, of the 248 included patients, 198 (79.8%) had successful healing without requiring amputation, while 50 (20.0%) required amputation to heal. The highest rate of amputation was observed in patients with bone involvement (I2 group). The healing results of the I0 and I1 groups were similar, while a significant difference was found between the I0 and I2 groups using the chi-square test (OR, 2.09; 95% CI, 1.08–4.04; P=0.027). Patients with positive bone culture results (I2 group) were found to have a risk of amputation 2.1 times higher than patients in the I0 group (Table 3). Additionally, the chi-square test revealed that the risk of amputation was significantly higher in the I2 group than in the I1 group, with an odds ratio of 3.07 (Table 4).
Discussion
Lower-limb amputation is often a consequence of soft tissue and bone infectious invasion. In patients with diabetes, infection is a significant predictor factor of amputation, and also an independent one [14]. Previous studies have shown that the risk of amputation in cases of severe DFI was more than 20% [14,15]. Patients with DFI have a significantly higher risk of requiring amputation than those without infection, with a reported 154-fold increased risk [16]. In addition, the concept of “silent infections” in diabetic foot ulcers has been suggested as a potential barrier to successful wound healing. Several factors have been suggested to indicate DFI when typical signs of infection are unclear. The presence of soft and easily breakable granulation tissue, slow healing despite proper wound management, and unexplained high blood sugar levels are factors that suggest the presence of DFI when typical signs are absent [10,17,18]. However, these symptoms may not be sufficiently distinct or have unclear indications from a clinical standpoint.
A previous study reported no association between bioburden without clinical signs of infection and healing outcomes in diabetic foot wounds [19]. However, there was a significant difference between their study and ours in terms of the methods used to obtain wound specimens: swab culture in the previous study and deep tissue biopsy in ours. Swab cultures are superficial, can be contaminated, and are not generally recommended for identifying pathogens in chronic wounds [20].
Early diagnosis and appropriate management are essential for the successful treatment of DFI. Therefore, our group performed tissue cultures for DFI diagnosis, even in cases without clinically overt infections. The optimal treatment involves sharp or surgical debridement, wound bed management, and effective antimicrobial therapy. Routine antibiotic therapy can lead to antimicrobial resistance, financial burden, and unexpected adverse events. Therefore, it should not be routinely administered to all patients with tissue-culture-positive wounds. Many experts do not recommend the use of antibiotics in clinically uninfected wounds. In our center, antibiotic use for DFI treatment is determined by various factors, such as the general condition of the patient, tissue perfusion, wound duration, and wound depth or size. If no clinical signs or symptoms of infection are present, and the tissue biopsy culture is negative, antimicrobial agents are not prescribed. Primarily, if only soft tissue culture is positive, local antimicrobial agents are administered along with sharp debridement. Systemic antibiotics are administered to patients with osteomyelitis.
Appropriate tissue perfusion is crucial for healing of diabetic wounds. Insufficient blood flow to the tissues can prevent wound healing and is a critical risk factor for major limb amputations. Severe PVD is strongly associated with a significantly higher amputation rate in patients with diabetes, with a reported 20-fold increase in risk [21-23]. Despite the I0 group having lower TcPO2 levels (31.5 mmHg) compared with the I2 group (37.3 mmHg), the outcomes of wound healing were found to be better in the I0 group compared with the I2 group, indicating that other factors affect the healing process. The findings of this study suggest that tissue culture can have a substantial effect on the healing outcomes of wounds that do not display any clinical signs of infection.
Interestingly, the I1 group showed better healing outcomes than the I0 group, although the difference was not statistically significant (84% in I0 vs. 88% in I1). This can be explained by the large TcPO2 difference between the two groups (31.5±18.8 mmHg in I0 vs. 45.6±21 mmHg in I1).
In this study, nearly half (49%) of the foot wounds showed positive tissue culture results, although they did not exhibit clinical signs or symptoms of infection. This discrepancy is significant because wounds that appear uninfected are not typically treated with antimicrobial medications. As previously stated, our team attempted to treat DFIs using tissue culture results. Nevertheless, the group with positive tissue culture results (group I2) showed a higher amputation rate. If the I2 group had been treated in the same manner as the I0 group without considering the positive tissue culture results, the amputation rate would have been higher than that observed in this study. Our study revealed an association between tissue culture results and healing outcomes of uninfected wounds. Preventing the advancement of infection based on tissue culture results has the potential to decrease negative consequences in people with diabetes.
In general, the positivity rate of soft tissue cultures is higher than that of bone cultures. According to previous studies, most DFIs are soft tissue infections, and approximately 20% are bone culture-related osteomyelitis [16,24]. However, in our study, the I1 group was much smaller than the I2 group (34 patients vs. 86 patients). Presumably, because our hospital is a tertiary center for diabetic foot ulcers, many advanced or severe cases were included in the study. Our patients usually had deep ulcers that may have already progressed to osteomyelitis.
According to the IDSA Clinical Practice Guidelines for DFI, the limited available evidence does not recommend the administration of antibiotics for treating clinically uninfected wounds, whether to enhance healing or as a preventive measure against clinically overt infections. In addition, they reported little evidence suggesting that subclinical infection or critical colonization might impede wound healing. We expect that the results of this study will provide evidence to support the importance of subclinical infections.
A strength of our study is that all included patients were thoroughly and uniformly managed by experienced wound specialists with long follow-up periods to determine their final wound-healing outcomes. However, the retrospective nature was one limitation of this study. Second, it relied exclusively on data collected during the initial intake exam. We believe that including data on the wound-healing progress and subsequent examinations might have resulted in confounding because of the difficulty of obtaining sufficient data. One study noted that initial wound grade was strongly related to the possibility of amputation [25]. Third, the much smaller size of the I1 group compared to the other groups could have led to statistical errors. In addition, a selection bias may exist in this study. The generalizability of our findings to other healthcare settings may be limited as our study included patients treated at a tertiary center. Finally, as the study did not include healing time, future studies are needed to better analyze this aspect.
Diabetic foot ulcers can be a major burden on patients and the medical system. The treatment of these patients requires long-term access, and healthcare costs are high. Nevertheless, treatment results for diabetic foot ulcers are not always successful. To the best of our knowledge, no study has explored the correlation between tissue culture results and healing of diabetic wounds that show no clinical signs or symptoms of infection, with the aim of determining the need for tissue culture in such cases. We expect that our results will help wound specialists resolve this challenge; our study demonstrated that tissue culture results are associated with wound-healing outcomes, even in clinically uninfected wounds in patients with diabetes. In the management of DFI, obtaining tissue cultures may be necessary to enhance wound-healing outcomes, even in the absence of clinical signs or symptoms of inflammation.
Notes
No potential conflict of interest relevant to this article was reported.