A Comparative Study of Dichloromethane and Ethyl Acetate Root Extracts of Celosia trigyna: Phytochemical and Wound Healing Effect Analyses
Article information
Abstract
Background
Celosia species have been validated to possess antimicrobial, anti-inflammatory, and antioxidant potential. This study was thus designed to comparatively determine the effect of dichloromethane and ethyl acetate root extracts of Celosia trigyna on Wistar rat wounds created by 8 mm biopsy punches.
Methods
Phytochemical screening was performed on dichloromethane and ethyl acetate root extracts of C. trigyna. For animal studies, 25 adult female Wistar rats were randomly divided into five groups (group A-E; n=5). Group A served as the negative control, group B as a positive control, group C received dichloromethane extract (once/day), group D ethyl acetate extract (once/day), and group E was the vehicle control. All extracts and medication were administered topically. Wound area, wound contraction, period of epithelialization, macroscopic analysis, and histological examination were studied.
Results
Phytochemical screening showed that saponins and terpenoids were particularly high in dichloromethane extracts while ethyl acetate extracts were highly rich in terpenoids. The results of wound areas, wound contraction, periods of epithelialization, and macroscopic and histological analyses showed that dichloromethane extract, ethyl acetate extract, and penicillin-treated groups showed better remediation respectively in order of efficacy compared to the negative and vehicle control groups. The histological examination revealed an evidence of tissue regeneration and collagen deposition in the extract-treated groups compared to the positive, negative, and vehicle control groups.
Conclusion
In conclusion, both extracts of C. trigyna have a better healing potential compared to penicillin treatment with dichloromethane extract having a better wound healing potential than the ethyl acetate extract.
Introduction
A wound is an injury to living tissue which can disrupt the normal anatomical structure and function of the tissue and its surrounding environment [1]. Wounds occur as a result of physical, thermal, chemical or microbial damage to the tissues [2]. The damage can affect the skin epithelial layer and can also extend into the subcutaneous tissues such as nerves, muscles and tendon thereby disrupting other structures [1,2]. The process of tissue repair after an injury to the tissue is referred to as wound healing. The wound healing process involves four phases, they are: hemostasis, inflammation, proliferation, and remodeling phases [3]. The duration of the wound healing process is dependent on many factors which include availability of biochemical substances required for each phase to occur, the extent of the tissue damage and the type of wound healing agent used. In modern medicine, orthodox medications are used in the treatment of wounds to increase the rate of the healing process and prevent contamination of the wound by microorganisms [4]. However, some of these medications have side effects, and sometimes wound contaminating bacteria are resistant to the effects of these orthodox medicines [5].
remediation due to their ethno-medicinal value and phytochemical potential. This has been one of the reasons why over 80% of the world population depends mainly on plants and plant extracts for their health care [6]. Research shows that medicinal plant extracts including some Celosia species are adopted in traditional treatment of wounds. For instance, Agyare et al. [7] concluded that the leaf, stem bark, and root extracts of Kigelia africana and Strophanthus hispidus exhibited antimicrobial, antioxidant, and enhanced wound healing properties and these may justify the medicinal uses of the plants for treatment of microbial infections and wounds. Also, investigation on Pupalia lappacea showed that it has good wound healing and antibacterial activities [8]. One of the Celosia species−Celosia argentea−has been documented to aid the healing process by promoting cell motility and proliferation of primary dermal fibroblasts [9]. However, even though C. trigyna has also been traditionally adopted for wound healing treatment [10], there is dearth of information on the scientific bases for such ethno-medicinal application.
C. trigyna is a medicinal plant of the family Amaranthaceae, found in Nigeria, South Africa, Southern Arabia, and the Democratic Republic of Congo. It is predominant in the Western and Northern parts of Nigeria. Due to its affinity for water, it is often cultivated during the raining season. It is soft, flavored, and easy to cook. C. trigyna is used for numerous medicinal purposes, such as in the treatment of intestinal worms, chest complaints, diarrhea, costal pains and mouth sores [11]. The leaves and roots are used to treat sores and skin problems [12,13]. The leaves are good sources of calcium, phosphorus, iron, protein, and vitamins A, C, and E [13]. Phytochemical analysis of species of Celosia shows they contain secondary metabolites such as triterpenoids, saponins, alkaloids, phenols, tannins, flavonoids, cardiac glycosides, steroids, and phlobatannins [14]. Despite the medicinal values of C. trigyna, the wound healing potential of the root extract has not been explored. This study is therefore set to comparatively determine the effect of dichloromethane (DCM) and ethyl acetate (EtOAc) root extracts of C. trigyna on wounds created by 8 mm biopsy punches on adult Wistar rats.
Methods
Plant material
The C. trigyna was collected from the southwestern part of Nigeria and authenticated by a taxonomist at the Department of Botany where a voucher specimen was deposited at the herbarium (Voucher number: IFE-17466). The roots of the plants were chopped into bits and air dried.
Chemicals and materials
DCM and EtOAc were procured from Sigma-Aldrich while Vaseline (Unilever), neurogesic ointments (Asset Pharmacy), penicillin (Anhui Medipharm Co., Ltd.), ketamine (Delphis Pharma), and biopsy punches (8 mm; Robbins Instruments) were procured from certified pharmaceutical outlets. Other materials used include rechargeable clippers and cotton buds. The chemicals and reagents used in this study were of analytical grade.
Extraction processes
The dried roots were pulverized with a crushing machine (Daiki Rika Kogyo Co., Ltd.). Thereafter, 1 kg of each of the crushed samples was extracted consecutively with DCM and EtOAc by continuous shaking on an orbital shaker for 48 hours. The extracts were filtered and concentrated using a rotary evaporator (Buchi Rotary Evaporator). The concentrated extracts obtained were stored in the refrigerator at 4 °C until required.
Phytochemical screening
The phytochemical screening of the DCM and EtOAc extracts was done by adopting the method by Rahman et al. [15].
Experimental animals
Twenty-five female Wistar rats (150–250 g) were used in this study. The rats were housed in animal holdings and acclimatized for 2 weeks before the commencement of the study. Ethical clearance (IPH/OAU/12/2031) was obtained from the ethical committee of the Institute of Public Health, Nigeria before the commencement of the research work. The animals were fed with standard pellet diet (Caps feed) and given water.
Preparation of ointments
Two ointments were prepared from the DCM and EtOAc extracts using petroleum jelly as a base (Vaseline). The Vaseline was melted in an oven (Charles Hearson & Co.) at 37 °C and mixed separately with the two extracts under continuous stirring until a homogenous mixture was obtained in the form of pomade.
Experimental design
The 25 rats were randomly assigned into five groups with five animals each: group A, negative control (no treatment); group B, positive control penicillin (once daily); group C, DCM extract (once daily); group D, EtOAc extract (once daily); or group E, vehicle control Vaseline (once daily). The treatment went on for 16 days.
Excision wound model
Each rat was weighed and anesthetized using ketamine (75 mg/kg) via intraperitoneal injection. The fur from the dorsal side was trimmed using scissors, then shaved using an electric clipper and shaving stick. Thereafter, the exposed areas were sterilized with 70% ethanol prior to wound infliction. A biopsy punch (8 mm) was used to induce wounds on the dorsal skin of the animals. This was a full-thickness wound and the depth of the excised wound included the panniculus carnosus muscle with the fascia forming the base of the wound. Neurogesic ointment was used as a pain killer and applied immediately after wound induction according to Qureshi et al. [16]. Twenty-four hours after wound creation, the ointments made from the two extracts, Vaseline and penicillin were applied topically once daily, according to the respective groupings as described above, to cover the wound site with the aid of cotton buds. Wound area, contraction, and epithelialization period were monitored [17]. Twenty-four hours after day 16, the animals were anesthetized via chloroform inhalation and the wound sites were excised and fixed in 10% formol saline.
Wound area
Wound area was determined every four days using acetate paper. The size of the wounds was traced on the acetate paper using a permanent marker and the diameter of the wound size was determined using a millimeter ruler. Thereafter, the area of the wound was calculated using the formula πd2/4, where d=diameter and π=3.14.
Wound contraction
Wound contraction was measured as a percentage contraction every 4 days until the 16th day of the study [17,18].
Macroscopic analysis
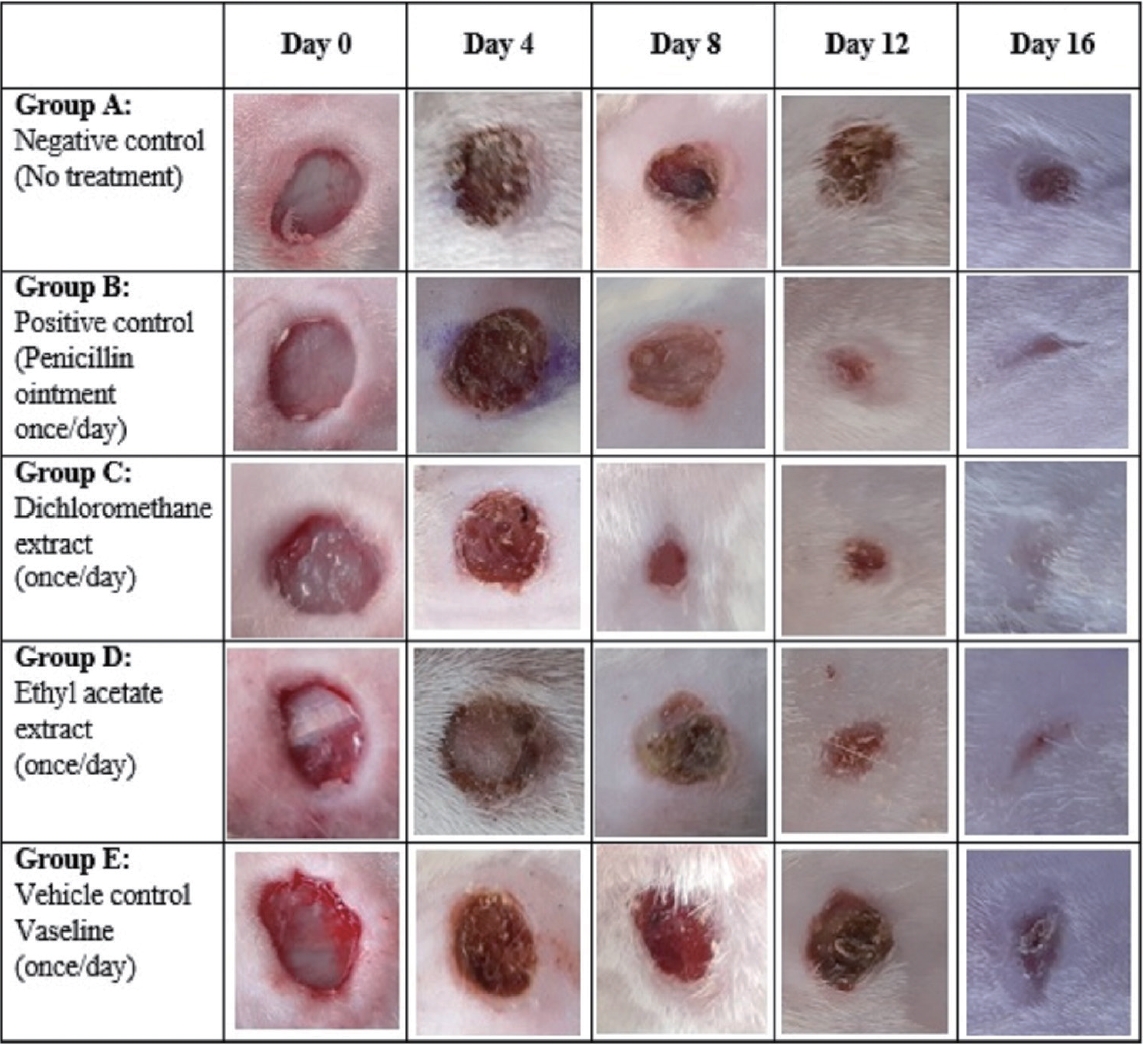
The wound sites were captured at equidistance of 15 cm from the tissue specimen across the group using a digital camera (Samsung A30) on days 0, 4, 8, 12, and 16 of the study. The images were achieved for macroscopic view and analysis.
Epithelialization time measurement
The period of epithelialization was calculated as the number of days required for the dead tissue remnants to fall off without any residual raw wound [18].
Histopathological analysis/photomicrography
The fixed tissues were passed through graded alcohol, cleared in xylene and embedded in paraffin wax. Serial section at 5 μm were obtained on a rotary microtome (Leica RM2125 RTS) and stained with hematoxylin and eosin and Masson trichrome. The slides produced were viewed under a light microscope (Leica DM 750) and the images were achieved using the attached camera (ICC 50). The intensity of Masson’s trichrome (collagen deposit) was quantified using Image J software by measuring the mean gray value of pixel. High staining intensity is synonymous to lower mean gray value according to the settings.
Statistical analysis
Statistical analysis was performed using the statistical package SPSS version 17.0 (SPSS) with Duncan’s Multiple Range Test option. One-way analysis of variance was used to evaluate statistical significance of the differences between animal groups. A value of P≤0.05 was considered significant. Data were reported as mean±standard error of mean.
Results
Comparative phytochemical constituent screening of DCM and EtOAc root extracts of C. trigyna
The quantitative phytochemical screening showed that the DCM root extract of C. trigyna was positive for the presence of tannin, resins, saponins, phlobatannins, sterols, alkaloids and terpenoids while the EtOAc root extract was positive for the presence of tannin, resins, phlobatannins, sterols, alkaloids and terpenoids. Terpenoids were strongly present in both DCM and EtOAc root extracts. Saponin was strongly present in DCM extract but absent in EtOAc root extract. Resins and sterols were mildly present in DCM extract but weakly present in EtOAc root extract. Glycosides, flavonoids, phenols and carbohydrates were all absent in both extracts as shown in Table 1.
Wound area
The wound area showed consistent decrement from day 0 to day 16 across all the groups. The groups treated with DCM root extract, EtOAc root extract and penicillin ointment showed a better remediation and significant difference (P<0.05) in wound area when compared to the negative control and vehicle control. The group treated with DCM extract presented the lowest wound area when compared to other groups as shown in Table 2.
Wound contraction and macroscopic analysis
The percentage of wound contraction was determined across the groups and presented in Fig. 1. There was no evidence of wound contraction on day 0 being the day of wound induction. As the days progressed, there were evidences of wound contraction starting from day 4 to day 16. There were similar trends in percentage wound contraction in the DCM root extract, EtOAc root extract and positive control treated groups. On day 16 which was the last day, the DCM extract treated group showed 96.55% wound contraction compared with 95.62%, 92.99%, 89.99%, and 63.76% for EtOAc, positive control, vehicle control, and negative control treated groups respectively as illustrated in Fig. 1. The results of wound contraction were also corroborated by macroscopic imaging of the wounds from day 0 to day 16 in each of the groups as shown in Fig. 2 and Supplementary Fig. 1.
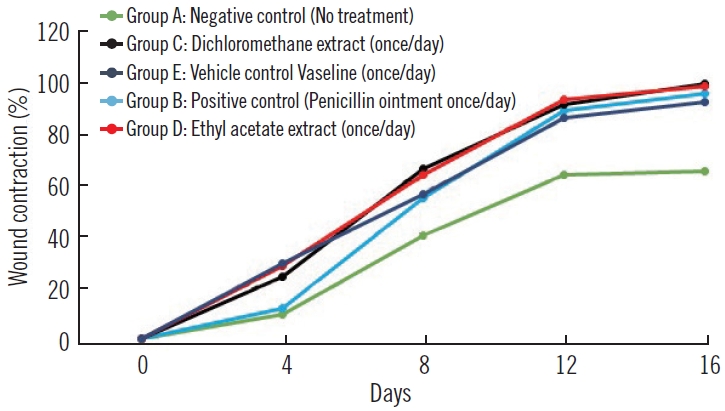
Percentage of wound contraction. Group A, negative control (no treatment); group B, positive control penicillin (once daily); group C, dichloromethane extract (once daily); group D, ethyl acetate extract (once daily); group E, vehicle control Vaseline (once daily).
Period of epithelialization
The period of epithelialization was faster in positive control, DCM extract, and EtOAc extract treated groups. The period of epithelialization was not significantly different (P>0.05) in the three groups. At the time of animal sacrifice, the negative (group A) and vehicle control (group E) groups were yet to reach the period of epithelialization and thus have no data as shown in Fig. 3.
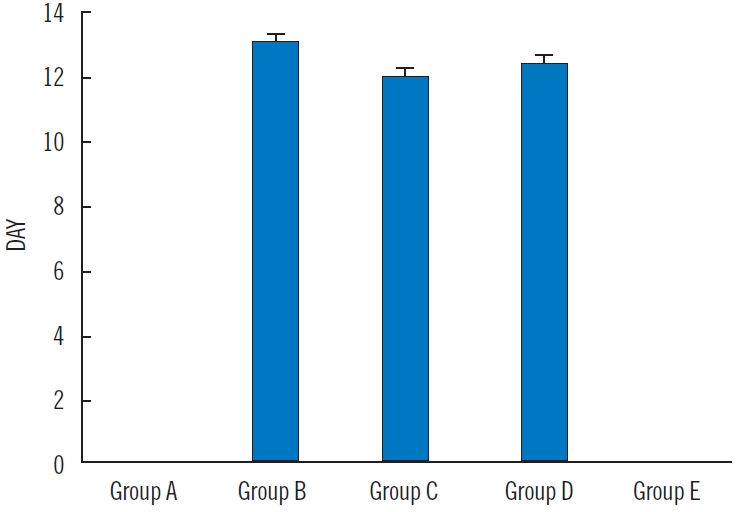
Period of epithelialization. Values are presented as mean±standard error of mean for period of epithelialization across the groups. There was no significant difference across groups B, C, and D at P=0.05 (using one-way analysis of variance with Duncan multiple range test). Group A, negative control (no treatment); group B, positive control penicillin (once daily); group C, dichloromethane extract (once daily); group D, ethyl acetate extract (once daily); group E, vehicle control Vaseline (once daily).
Histopathological examination
Hematoxylin and eosin stain
The histological outline results of the groups are presented in Fig. 4. The well-aligned stratified squamous keratinized epithelia is an evidence of complete regeneration of the epidermis in the DCM root extract (Fig. 4C), EtOAc root extract (Fig. 4D), and positive control treated groups (Fig. 4B) when compared to the negative control (Fig. 4A) and vehicle control treated groups (Fig. 4E) which were characterized by sinusoidal pattern of the epithelial layer of the epidermis. The dermis of the skin in all groups showed evidence of budding new blood capillaries as shown in Fig. 4.
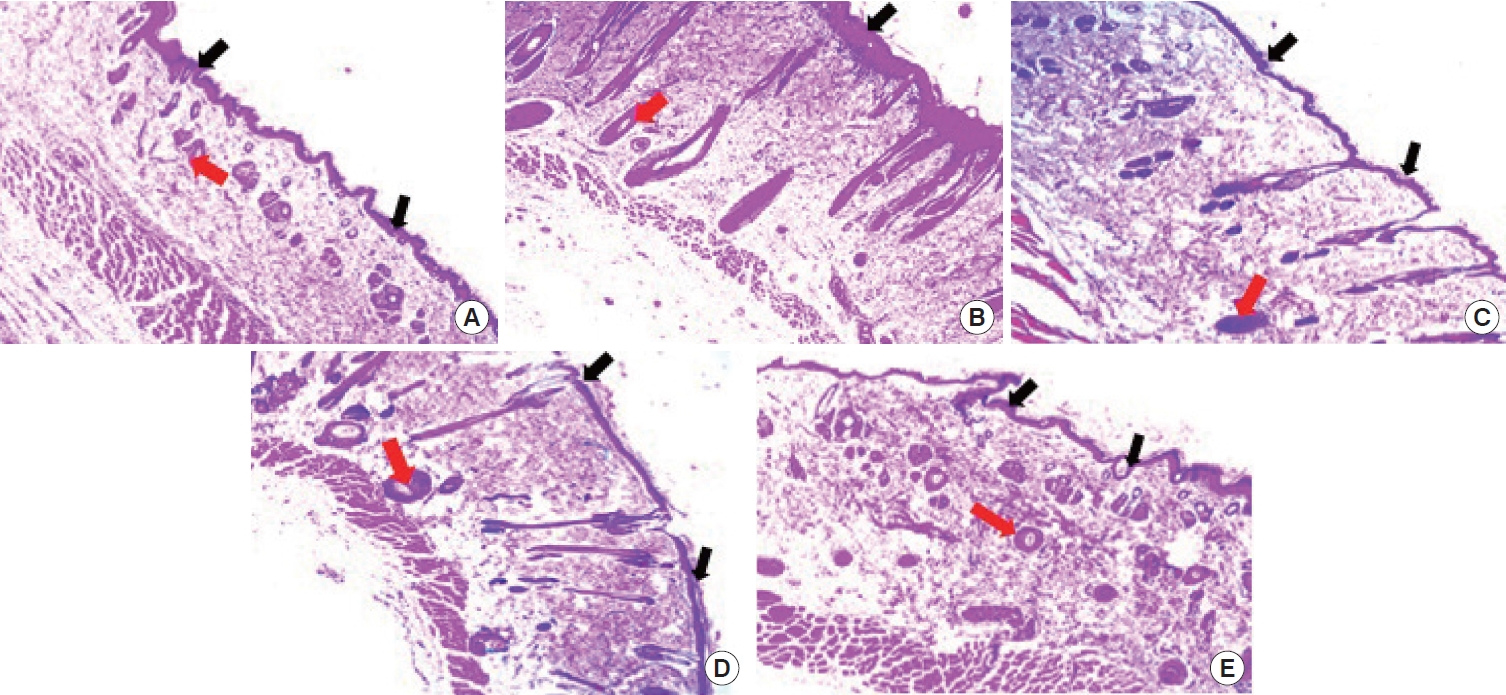
Photomicrographs of the skin. (A) Negative control. The epidermis appears sinusoidal (black arrows), evidence of incomplete regeneration process. Few blood capillaries (red arrow) were observed within the dermis. (B) Positive control. The epidermis presented a thick and smooth outline (black arrow) compared to panel A. Numerous budding new capillaries (red arrow) were visible within the dermis of the skin. (C) Dichloromethane extract group. The epidermis appears thinner (black arrows) compared with panel B but similar to panel D. The dermis was dominated with numerous blood capillaries (red arrow). (D) Ethyl acetate extract group. The epidermis appears thinner (black arrow) similar to panel C with thin epidermis and numerous blood capillaries (red arrow) within the dermal layer of the skin. (E) Vehicle control. The epidermis (black arrow) appears sinusoidal in nature which is similar to panel A. The dermis is studded with few blood capillaries (red arrow). Hematoxylin and eosin staining, ×40.
Masson trichrome stain
The Masson trichrome stain is specific for collagen fibers. The results from Fig. 5 show moderate distribution of collagen fibers in negative (Fig. 5A) and vehicle control (Fig. 5E). This was corroborated by high mean gray values which correspond to low staining intensity for collagen fibers. There were high distribution of collagen fibers in the positive control (Fig. 5B), DCM (Fig. 5C), and EtOAc (Fig. 5D) treated groups. This observation was supported with the low mean gray values which correspond to high staining intensity for collagen fiber distribution as observed in this study. The mean gray values of the positive control, DCM, and EtOAc treated groups were not significantly different (P>0.05) across the groups but differ significantly when compared with the negative and vehicle control groups as shown in Fig. 6.

Photomicrographs of the skin. Collagen fibers stain green. (A) Negative control. Minimal deposition of collagen fibers. (B) Positive control. There was moderately high deposition of collagen fibers similar to panel C. (C) Dichloromethane extract group. There was high deposition of collagen fibers which was the most significant among the groups. (D) Ethyl acetate extract group. There was moderately high deposition of collagen fibers similar to panel C. (E) Vehicle control. There was moderate deposition of collagen fibers similar to panel A. Masson trichrome staining, ×40.

Mean gray values. Values are presented as mean±standard error of mean for mean gray values across the groups. a),b)Across groups signifies that means with different letters differ significantly at P=0.05 while means with the same letters do not differ significantly at P<0.05 (using one-way analysis of variance with Duncan multiple range test).
Discussion
Many medicinal plant extracts have been tested for their efficacy in wound healing [6,19]. In certain circumstances, alternative medical approaches are being preferred over modern medicine due to fewer side effects and lesser toxicity [20]. Sandhya et al. [21] posited that wound healing plant products are easy to administer and are extensively available. Despite documentations on the traditional use of C. trigyna in wound healing, the scientific bases are yet to be substantiated. The results from this study showed a non-significant difference in wound area between the positive control, DCM, and EtOAc groups on day 16 of the study, with the DCM group having the best wound healing potential as reflected in the reduced wound area compared to all other groups.
This observation was also replicated in the wound contraction and macroscopic analysis of this study. Getie et al. [22] established the rationale between the efficacy of a medication and wound contraction. The greater the percentage wound contraction, the better the efficacy of such medication. The efficacy of both the DCM and EtOAc extract treated groups were justified by their ability to significantly increase the percentage wound contraction thus fast-tracking the period of epithelialization as observed in this study. The accelerated healing potential of both DCM and EtOAc root extracts may be due to the induction of macrophage cell proliferation or presence of important antibacterial and phytochemicals properties that are believed to promote wound healing [23]. The phytochemical screening of both DCM and EtOAc root extract showed the presence of tannin, resins, saponins, phlobatannins, sterols, alkaloids and terpenoids in various proportion. Saponins and terpenoids were particularly high in DCM extracts while EtOAc extracts were highly rich in terpenoids. Saponins have been established to have high healing potentials [6,19] while terpenoids, through their antimicrobial activities, have been able to bring about increased rate of epithelialization and wound contraction [24].
The healing potential of C. trigyna must have justified the reasons for increased rate of epithelialization and wound contraction in the DCM and EtOAc root extracts compared to other treated groups including the positive control group. More studies have corroborated the healing efficacy of saponins and phlobatannins which are present in the DCM and EtOAc root extracts and by extension may have played a significant role in the wound-healing process.
The histopathological analysis results showed evidence of complete regeneration of the epidermis and budding of new blood capillaries in positive control, DCM and EtOAc groups when compared with the ongoing regeneration of the epidermis noticed in negative and vehicle control. The DCM and EtOAc groups showed a similar outlook in the regenerative processes. The collagen distribution in the treated groups showed more collagen deposit in positive control, DCM and EtOAc treated groups when compared to the negative and vehicle controls. Quantitative assessment of collagen deposits corroborate the qualitative (histological) assessment. There was a non-significant difference in the mean gray value between the positive control, DCM and EtOAc treated groups in this study. However, there was a significant increase in collagen deposit in the DCM and EtOAc treated groups when compared to the negative and vehicle control groups. Collagen deposition has been posited to be an important event that occurs during wound healing process [20]. During wound healing, there is a gradual process that involves the biosynthesis and deposition of new collagen and subsequent maturation [20,25]. The DCM and EtOAc root extracts of C. trigyna may have increased collagen deposit through its earlier documented pharmacological potentials [14]. Studies have shown that Celosia species possess antioxidant, anti-inflammatory, wound healing and antibacterial activities [14]. All these properties are what is dominant in a potent, conventional wound healing interventions.
In conclusion, the DCM root extract treated group showed the best remediation in different phases of wound repair such as wound area, wound contraction, period of epithelialization, tissue regeneration and collagen deposit. This was closely followed by EtOAc and positive control groups in order of efficacy. Evidence from this study further validates the traditional use of C. trigyna for treatment of wounds and that the roots are greatly endowed with significant wound healing properties.
Notes
No potential conflict of interest relevant to this article was reported.
Supplementary material
Supplementary Fig. 1.
Macroscopic analysis of wound sites for all animals on the final day.
Supplemental data can be found at: https://doi.org/10.22467/jwmr.2023.02411.
jwmr-2023-02411-Supplementary-Fig-1.pdf