Reconstruction of Upper Extremity Using Pedicled Latissimus Dorsi Myocutaneous Island Flap and Skin Graft after Necrotizing Fasciitis: A Case Report
Article information
Abstract
Necrotizing fasciitis of the upper extremities is a severe, life-threatening condition, potentially resulting in amputation or death in the absence of prompt and aggressive surgical treatment. Reconstruction is eventually required after adequate surgical debridement, and patients are often referred to a plastic surgeon for this purpose. Herein, we present the case of a patient with extensive soft tissue defects on the upper extremity after fasciotomy for necrotizing fasciitis. We successfully performed a pedicled latissimus dorsi myocutaneous island flap, full-thickness skin graft, and split-thickness skin graft for this patient. After 2 months, the patient did not have any wound problems and could carry out adequate upper extremity motion.
Introduction
Necrotizing fasciitis is a severe disease which can be life-threatening, involving widespread and rapid soft tissue inflammation through the fascia. Occurring mainly after surgery or trauma, necrotizing fasciitis can develop in any body part, but primarily occurs in the extremities [1,2]. If prompt and aggressive surgical treatment is not performed, necrotizing fasciitis can lead to death. The mortality rate varies among studies but is estimated to be between 16% and 30% [3]. Because the lesion spreads rapidly through the fascia, the defect site can be very large after infection is controlled. Amputation is reported in about 22% of cases [4,5].
A case of upper extremity necrotizing fasciitis occurred in a 72-year-old male patient with a history of hypertension and diabetes, and we herein present the reconstruction of the resulting defects. Since there were extensive defects from the shoulder to the upper arm and forearm, both functional and cosmetic aspects had to be considered. Our case was noteworthy particularly because of the close cooperation and collaboration of multiple departments of our hospital. From admission to the intensive care unit, general physicians took care of vital signs and the patient’s septic condition. The orthopedic surgeons performed emergency debridement, after which reconstructive plastic surgeons conducted wound coverage. The department of infectious disease decided on adequate antibiotic usage. Such process of treatment in this case provides valuable information for managing comparable patients.
Regarding the wound of our patient, the muscles and tendons were exposed, and a free flap could be considered as one of the coverage options. However, due to the patient’s old age and underlying diseases, a lengthy operation would have posed risks, and it was impossible to cover all the defects with one free flap. Therefore, another coverage method was needed. It was believed that some defects could be reconstructed with a pedicled latissimus dorsi (LD) myocutaneous island flap, which would not require microsurgery. However, it was impossible to cover the multiple wounds with such a flap [6,7]. Therefore, for this case of extensive defects ranging from the shoulder to the upper arm and the forearm, we present a reconstruction method using a pedicled LD myocutaneous island flap and skin grafts.
The report was approved by the Institutional Review Board of Konkuk University Hospital (IRB No. 2022-08-004). Written informed consent was obtained from the patient.
Case
A 72-year-old male patient with a history of hypertension and diabetes visited the emergency room of our hospital due to redness, pain, and skin color change in the left upper extremity after a fall-down accident caused by dizziness 1 week prior (Fig. 1). Extensive emphysematous subcutaneous edema and intermuscular fascia necrosis from the left shoulder to the forearm were observed on upper extremity computed tomography. Additional extensive soft-tissue emphysema in the anterior chest wall and deltoid muscle were found, prompting a diagnosis of necrotizing fasciitis (Fig. 2). We relied on the laboratory risk indicator for necrotizing fasciitis (LRINEC) score to quickly determine the diagnosis. In our case, the laboratory findings were as follows: C-reactive protein (CRP) >32 mg/dL; white blood cell count, 6,440 cells/μL; hemoglobin, 13.5 g/dL; sodium, 133 mmol/L; creatinine 0.89 mg/dL; and glucose, 317 mg/dL. These findings corresponded to a total LRINEC score of 7.6. This was considered to indicate a moderate risk of necrotizing soft-tissue infection.

Rapid aggravation of necrotizing fasciitis. (A) Initial clinical photograph at the emergency room. Tissue necrosis involved upper arm and medial elbow area. (B) Necrotizing fasciitis progressed to the cubital fossa and forearm areas on the 3rd day of hospitalization.
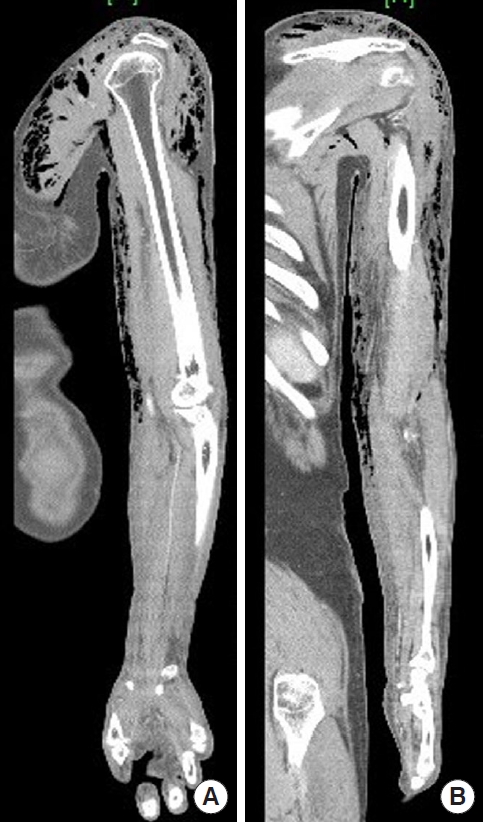
Imaging study of the tissue necrosis. Extensive subcutaneous emphysema and fascia necrosis from the left shoulder to the forearm were noted on upper extremity computed tomography: (A) sagittal and (B) coronal views.
Due to the possibility of septic shock and the high risk of mortality, an orthopedic surgeon decided to perform an emergency fasciotomy after the patient’s admission to the intensive care unit. As the first empirical antibiotic, Tazoperan (4.5 g) was administered four times a day, and clindamycin (0.9 g) was administered three times a day. After confirming that Klebsiella pneumoniae grew in the wound and blood cultures, the antibiotic therapy was changed to ceftriaxone. Three debridement procedures were performed including the emergency fasciotomy. Subsequently, along with the recovery of the patient’s overall general condition, the CRP level was significantly reduced to 1.66 mg/dL, and wound discharge and tissue sloughing mostly improved.
The patient was referred to a plastic surgeon to reconstruct extensive tissue defects from the left shoulder to the upper arm and forearm that occurred after debridement. A reconstruction plan was established for the soft tissue defects in the anterior shoulder, posterior shoulder, upper arm, medial elbow, and circumferential forearm area (Fig. 3).

Clinical photographs before reconstruction after serial debridement. (A) Upper arm to the forearm, (B) shoulder to the upper arm, and (C) posterior area.
Due to hypertension, diabetes, and recent general anesthesia, prolonged surgery was considered risky. Postoperative wound care for the entire upper extremity was thought to be difficult after single-stage reconstruction. Therefore, it was decided to divide the reconstruction procedure into two stages. We also concurred that the two-stage reconstruction would help cover some of the multiple defects more safely and efficiently than covering them with free flaps.
The primary coverage operation was performed 17 days after the initial debridement. First, the upper arm was covered as much as possible with a pedicled LD myocutaneous island flap. For this, left LD flap elevation (about 20×9 cm) was performed, and the proximal part was skeletonized along the pedicle. The LD flap was transferred toward the upper arm and medial elbow defects through a tunnel made in the axillary soft tissues (Fig. 4). The medial elbow area is a joint where muscle origins are located. Afterwards, local advancement flap coverage was performed for a posterior shoulder area defect measuring 15×5 cm. The total operation time was about 5 hours.
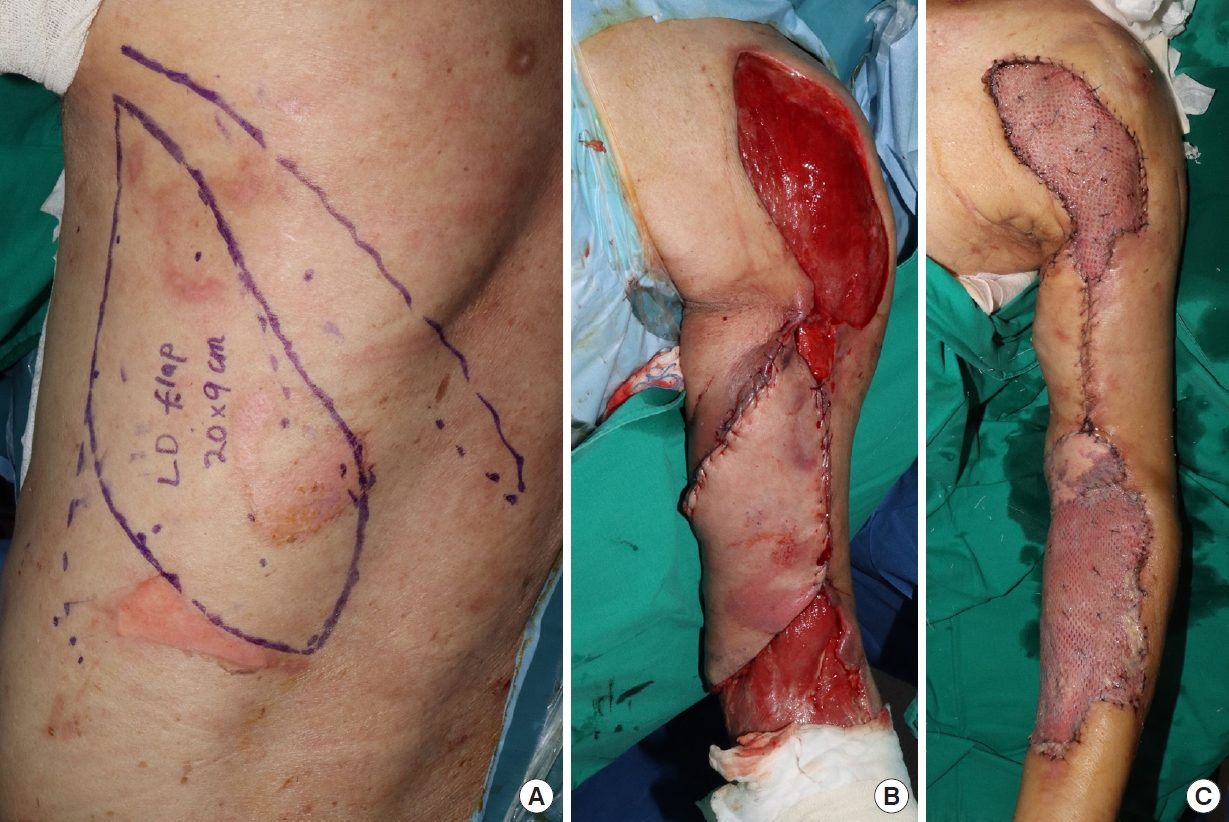
Clinical photographs before reconstruction after serial debridement. (A) Upper arm to the forearm, (B) shoulder to the upper arm, and (C) posterior area.
One week after the first surgery, the remaining defect areas were covered with skin grafts. Since the cubital fossa is a vulnerable area where blood vessels and nerves are concentrated, it was decided that a thick skin layer would be needed for a functional range of motion and prevention of secondary infection. Using the lower abdomen area as the donor, a full-thickness skin graft of about 10×5 cm was performed. For the anterior shoulder and the circumferential forearm areas, a split-thickness skin graft was performed along with a Matriderm graft on the wrist. As flexor tendons are located in the wrist, Matriderm was applied for improved excursion of the tendons. The measured extent of each graft surface was 15×8 cm on the shoulder and 20×10 cm on the forearm; both were harvested from the left thigh. The thickness of the split-thickness skin grafts was 0.3 mm, and the skin was expanded into a 1.5:1 meshing ratio for efficient coverage. The total operation time was about 3 hours. As for postoperative antibiotics, intravenous ceftriaxone was administered until the 7th day after the skin graft procedure. The antibiotic treatment was based on the preoperative K. pneumoniae growth in wound swab culture.
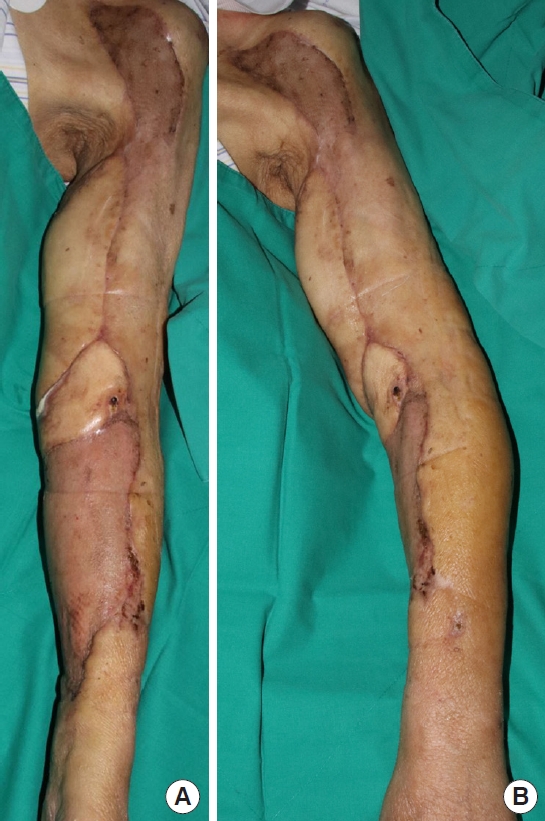
Two months after surgery, the flaps and skin grafts were all well taken. There were no wound problems (Fig. 5). Regarding the range of motion of the elbow joint, extension was possible up to 0° (normal: 0°) and the patient could perform active flexion to 90° (normal: 150°). The range of pronation was 90° (normal: 90°) and the range of supination was 10° (normal: 90°) respectively when the elbow was flexed at 90°.
Discussion
As necrotizing fasciitis leads to significant tissue defects, it requires active surgical debridement in initial treatment. After the patient’s general condition is stabilized, the next concern of surgeons is defect coverage. Various operative methods have been presented to restore wounds from necrotizing fasciitis of the upper or lower extremities [5,6,8]. The reconstruction of extensive soft-tissue defects in the upper extremity is challenging due to the unavailability of expendable local muscle. Therefore, the method of reconstruction should be determined considering the patient’s overall general condition, the defect site, and functional and cosmetic purposes.
In our case, even if a free flap was performed, it would not have been possible to cover all the defects with a single flap, and prolonged surgery would have been risky to the patient. This case is meaningful in that extensive upper extremity defects were reconstructed by appropriately dividing a pedicled LD myocutaneous island flap, full-thickness skin graft and split-thickness skin graft according to the specific area. The LD flap was applied on the medial epicondyle where the origins of various forearm muscles are located. The full-thickness skin graft was selected to cover the vulnerable cubital fossa as postoperative scar contracture of this area can be problematic as well [9].
In necrotizing fasciitis patients, it is important to determine the timing of defect coverage after debridement. If early coverage is performed in a situation where an infection still remains or wound bed preparation is insufficient, the possibility of coverage failure may increase. Furthermore, most necrotizing fasciitis patients have underlying diseases such as diabetes and hypertension, increasing the risk from prolonged surgery; free flap coverage is often impractical and the risk of flap failure is high [5].
LD flaps have been used for many years for breast reconstruction as well as for upper extremity reconstruction. Due to the reliability, predictable anatomy, and relatively easy dissection of LD flap, it has been preferred by plastic surgeons for the reconstruction of the upper extremities [6,7]. Compared with a pedicled LD flap, a free flap can efficiently construct convoluted soft tissue defects. However, it has the disadvantages of long operation time and the possibility of flap loss. Pedicled LD flaps had been previously utilized to cover various defects of the elbow and the distal third of the arm [7,10]. The main drawback of a pedicled LD flap is the limited arc of rotation. Nonetheless, meticulous skeletonization techniques were introduced in previous literature to overcome the limitation. Chateau et al. [7] noted the independent dissection of vessels and nerves adjacent to the origin of the circumflex scapular artery. Hacquebord et al. [10] reported the versatility of a pedicled LD flap to cover considerably sizeable soft tissue defects (100–147 cm2) around the elbow.
In necrotizing fasciitis patients, clinical features such as swelling, pain, and erythema of the affected area commonly present. In addition, bulla and skin necrosis can be exhibited [3]. Magnetic resonance imaging is the gold standard for diagnosing complicated skin and soft tissue infections, including necrotizing fasciitis. However, when necrotizing fasciitis is suspected at the emergency department, computed tomography is often used as a primary imaging modality because of its availability. Gas within fluid collections along the subfascial plane is the most significant characteristic of necrotizing fasciitis on computed tomography images [9].
Initial diagnosis of necrotizing fasciitis is not easy. According to Goh et al. [3], about 50% of cases are misdiagnosed. However, the LRINEC score can differentiate necrotizing fasciitis from other soft-tissue infections [11]. Meanwhile, though the LRINEC score may be a tool for rapid diagnosis, Crowe et al. [12] reported that it could not be used as a predictor of mortality or amputation, and that vasopressin dependence is a strong predictor of mortality.
Necrotizing fasciitis is classified into types according to the detected microorganism. In our case, only K. pneumoniae was detected, corresponding to a monomicrobial type. Monomicrobial necrotizing fasciitis has recently been increasing in incidence, more so than the polymicrobial type. Among monomicrobial necrotizing fasciitis, K. pneumoniae type has a mortality rate of up to 60%, and necrotizing fasciitis caused by K. pneumoniae has been reported to occur frequently in patients with diabetes [13,14].
The limitation of this study is the short follow-up period. The reconstructed wound was managed for 2 months after the LD flap operation. Afterward, the patient was transferred to another hospital for rehabilitation, and timely outpatient follow-up was not possible. Nonetheless, the patient’s condition was verified with a telephone interview, and adequate maintenance of function was noted.
Skin and soft tissue defects occurring after rapid and thorough debridement for upper extremity necrotizing fasciitis may be challenging as it is difficult to cover extensive defects with a single coverage method. In this case, extensive defects were covered appropriately with a combination of reconstructive options (e.g., pedicled LD myocutaneous island flap, full-thickness skin graft and split-thickness skin graft).
Notes
No potential conflict of interest relevant to this article was reported.