The Effect of Topical Normobaric Oxygen Therapy on Composite Graft Survival in Fingertip Amputation
Article information
Abstract
Background
Though composite grafting is an easy, simple treatment for fingertip amputation, it is known to have a low survival rate. To increase the likelihood of composite graft survival, various adjuvant therapies such as hyperbaric oxygen therapy, ice-cooling, or vasodilator agents have been used. In this study, we attempted to validate the hypothesis that topical normobaric oxygen therapy (tNBO) could improve composite graft survival in fingertip amputation.
Methods
Patients who sustained fingertip amputations and who were treated with composite grafting between January 2015 and July 2020 were included. The patients (n=154) were divided into two groups: those who received tNBO (n=102) and those who had not (n=52). The effect of tNBO on graft survival, survival rate by level of amputation, and risk factors of graft survival (age, smoking, time to surgery, diabetes mellitus, and crush-avulsion injury type) were examined.
Results
tNBO significantly increased composite graft survival (75.3% vs. 50%, P<0.001) in amputations distal to the nail base area. Among risk factors, time to surgery >5 hours (odds ratio, 48.6; P=0.001) and crush-avulsion injury type (odds ratio, 10.1; P<0.001) significantly decreased graft survival in both groups. Smoking decreased graft survival only in the non-tNBO group (odds ratio, 28; P=0.015), not in the tNBO-treated group.
Conclusion
tNBO increased composite graft survival in fingertip amputation distal to the nail base area. It can be helpful for composite graft survival in smokers.
Introduction
The fingertip is the area distal to the insertion of the flexor and extensor tendons of the distal phalanx [1,2]. Amputations of the fingertip can often result in severe functional and aesthetic damage; replantation is the treatment of choice. However, microvascular anastomosis is often impossible due to vessel injuries and the miniscule size of the end. In such instances, alternative treatment options, such as secondary intention healing, skin grafting [3], local flaps [4], microvascular free tissue transfer [5], and composite grafting [6,7] are considered. Composite grafting is frequently preferred because it is a quick an d easy procedure, and also preserves maximal finger length and natural shape.
A major issue with composite grafts is the low graft survival rate, which has been demonstrated to be 50% or less [8,9]. Age >18 years, smoking, time to surgery (>5 hours), underlying diseases (such as diabetes mellitus), and crush-avulsion-type injury are well-known risk factors that threaten composite graft survival [3,6,8,10]. Many studies have been performed to increase composite graft survival [5,7,10,11]. Son et al. [10] indicated that a humid environment can improve graft survival. Eo et al. [7] and Hirase [5] stated that ice-cooling and prostaglandin E1 (PGE1) can increase graft survival. Nichter et al. [11] demonstrated that hyperbaric oxygen therapy (HBO) has a positive effect on graft survival.
HBO promotes wound healing through tissue oxygenation [12,13]. HBO has been employed in osteomyelitis [14], radiation injury of bone and soft tissue [15], necrotizing soft tissue infection [16], ischemic wound healing [17], and composite grafts [11]. However, one constraint of HBO is that it can only be administered for a few hours per day because of oxygen toxicity [18]. It is also costly and requires a large space for the equipment.
Compared to HBO, topical normobaric oxygen therapy (tNBO), which topically supplies oxygen at sea-level pressure, is cost-effective because it can be performed in low-resource settings. It is also free from the risk of barotrauma and pulmonary toxicity. Animal studies have demonstrated that normobaric oxygen therapy can accelerate tissue oxygenation and enhance graft survival [12,19,20]. However, to the best of our knowledge, clinical studies regarding the effect of tNBO on composite grafts in fingertip amputation have not been reported. Therefore, the objective of this study is to validate the effect of tNBO on composite grafts in fingertip amputation.
Methods
Study design and participants
Patients were enrolled between January 2015 and July 2020. The inclusion criteria were as follows: (1) patients of all ages and both sexes; (2) patients with fingertip amputations distal to the distal interphalangeal joint (DIPJ); and (3) patients who were treated with composite grafting. The exclusion criteria were as follows: (1) patients with incomplete amputations where the amputated distal tip was partially connected to the proximal stump; (2) patients whose amputated distal tips were lost and salvage impossible because of severe crush injury; and (3) patients lost to postoperative follow-up. Written informed consent was obtained for publication of this study and accompanying images, and the study was approved by the Institutional Review Board of Kyung Hee University Hospital (IRB No. 2022-07-077).
Surgical approach
The amputated fingertip was immersed in normal saline and stored at 4 ºC until surgery. Operative techniques were consistent across all patients. Four plastic surgeons performed surgeries under either general or local anesthesia, depending on the severity of the injury, the patients’ choice, and their age (i.e., most pediatric patients underwent surgery under general anesthesia). In cases of local anesthesia, a digital block of the finger was employed using a 2% xylocaine solution. The amputated distal tip and stump were disinfected using povidone iodine. Any remaining foreign substances were removed by vigorous irrigation using normal saline solution. All debris and dead tissue were removed. Invasive procedures such as debridement or de-epithelialization of the amputated distal tip were minimized. The amputated distal tip was anchored to the stump with 5-0 nylon sutures, focusing on aligning the edge of the skin. In nail bed injuries, nail bed repair was performed using 5-0 Vicryl sutures after nail extraction. Extracted nails were sutured back onto the nail bed if the nail was not too severely crushed. If the damage was too severe, an artificial nail was used. When a distal phalanx fracture was present, bone fixation was performed with a Kirschner wire. Dressings were subsequently performed using a minimum amount of antibiotic ointment and a gas-permeable foam to avoid restricting oxygen permeability. Following surgery, the entire hand and distal arm were fixed for 2 weeks using a splint. Antibiotics administered varied from first-generation cephalosporins to triple antibiotics based on the status of the wound. The antibiotics were used for a maximum of 1 week after surgery. Ten milligrams of lipo–PGE1 (Eglandin, Welfide) in 5% dextrose solution were infused for 4 hours per day over 5 days after surgery.
Topical normobaric oxygen therapy
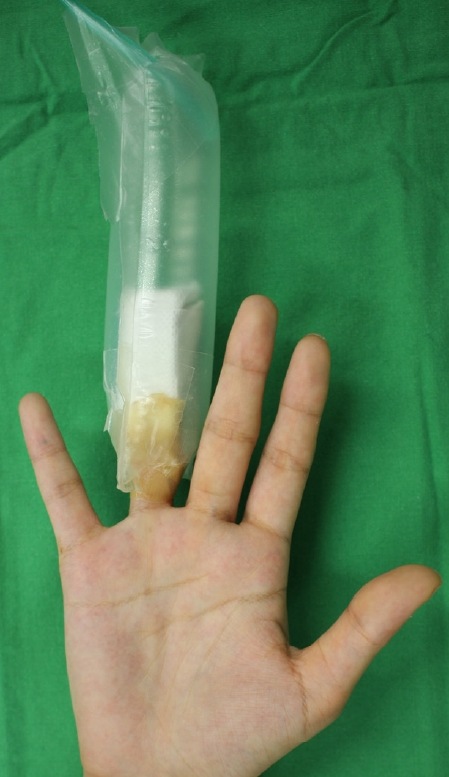
tNBO was administered based on patient’s consent and surgeon’s choice. tNBO was administered immediately after surgery and maintained all day for 7 to 10 days. The injured site was sealed using a plastic bag and transparent adhesive film dressing (Opsite, Smith & Nephew). A flexible oxygen tube was inserted into the plastic bag, and the opposite end of the tube was connected to an oxygen generator on the wall of the ward (Fig. 1). Multiple 18-gauge needle-sized holes were made for oxygen flow to escape the plastic confines. The flow rate, determined empirically, was 2–3 L/min. The seal was re-applied immediately after each wound dressing treatment. Patients were instructed to stay connected to the oxygen generator for as long as possible. While performing tNBO, a humidifier was used to prevent dryness.
Data collection procedure
Data were collected from medical records and clinical photographs of the enrolled patients. Age, sex, injury type, amputation level, body mass index, smoking (at least once in the month before admission), underlying disease (diabetes mellitus), time to surgery, and graft survival were analyzed. Patients were divided into two groups: tNBO-treated group (NBOG) and non-tNBO-treated group (NNBOG).
Levels of amputation were analyzed using the Ishikawa classification [21]: subzone I, from fingertip to midway between the fingertip and nail base; subzone II, from midway between the fingertip and nail base to the nail base; subzone III, from nail base to midway between the nail base and DIPJ; and subzone IV, from midway between the nail base and DIPJ to the DIPJ. When fingertips were amputated obliquely, the center point of the stump was used as the criterion for assessment.
Type of injury was assessed using the Biemer classification [22]: (1) clean-cut, resulting from a sharp object with no tissue loss and minimal crushing of the tissue; (2) blunt-cut, resulting from a blunt object with minimal loss of tissue and crushing of the tissue; and (3) crush-avulsion, resulting from severe crushing or avulsion of the tissue (Figs. 2, 3).
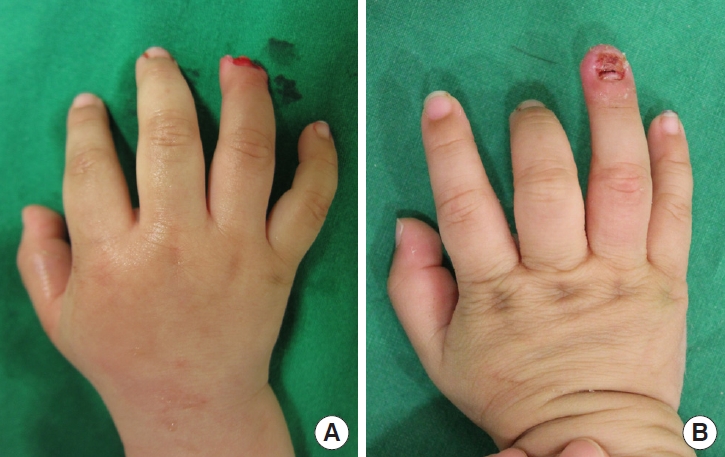
Case 1 of crush-avulsion amputation. (A) A 1-year-old male patient who sustained crush-avulsion amputation on the right 4th finger in subzone III by a car door. After surgery, topical normobaric oxygen therapy was applied to the finger for 1 week. (B) Final result: 3 weeks postoperatively. The composite graft survived completely.
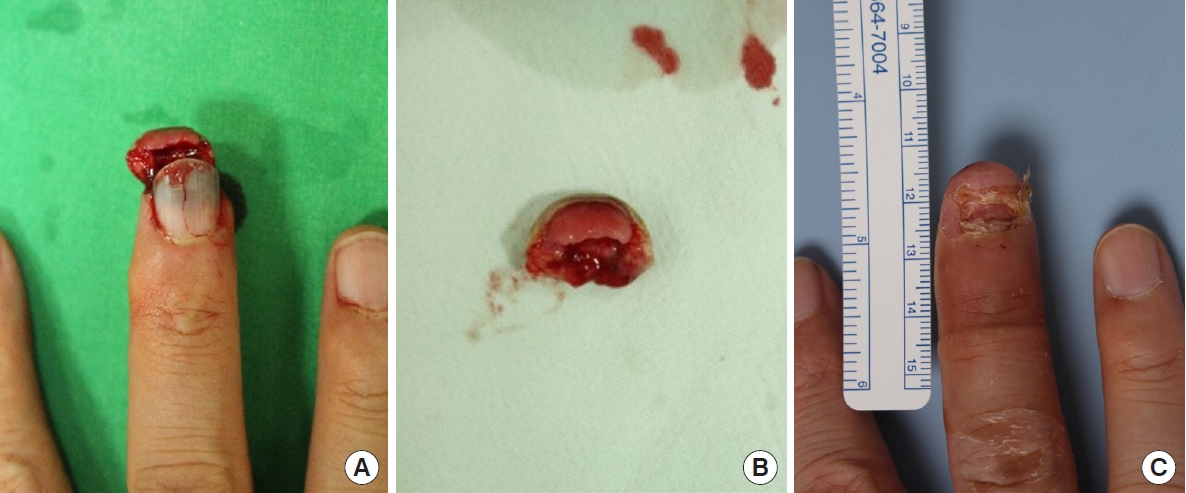
Case 2 of crush-avulsion amputation. (A) A 48-year-old male patient who sustained oblique crush-avulsion amputation of the left middle finger in subzone II by a machine. He had smoked cigarettes for 20 years. After surgery, topical normobaric oxygen therapy was applied to the finger for 1 week. (B) Dorsal view of the amputated fingertip. (C) Final result: 1 month postoperatively. The composite graft survived completely.
Graft survival was assessed 2 weeks after the surgery. Following the definition of Heistein and Cook [8], complete survival was defined as <10% necrosis, partial survival was defined as 10% to 50% necrosis, and no survival was defined as >50% necrosis. Follow-up was performed until 6 months after the surgery.
Statistical analysis
Statistical analysis was performed using SPSS software for Windows version 15.0 (SPSS Inc.). The independent t-test and chi-square analysis were employed. Graft survival was analyzed by linear association analysis. The effect of risk factors on graft survival was evaluated by logistic regression analysis. Statistical significance was considered at P<0.05.
Results
Patient demographics
Among the 202 patients, 48 were excluded. Of the 154 patients included in the study (161 fingertips including five patients with 2-finger injuries, and one patient with 3-finger injuries), 102 (109 fingertips) were NBOG and 52 (52 fingertips) were NNBOG. The characteristics of the fingertips are summarized in Table 1.
Composite graft survival
The complete graft survival rate was 66.7%. Complete survival rates for NBOG and NNBOG were 75.3% and 50.0%, respectively. Linear ordinal trend analysis revealed that tNBO significantly increased the composite graft survival rate (P<0.001) (Table 2).
Evaluation of composite graft survival based on amputation level
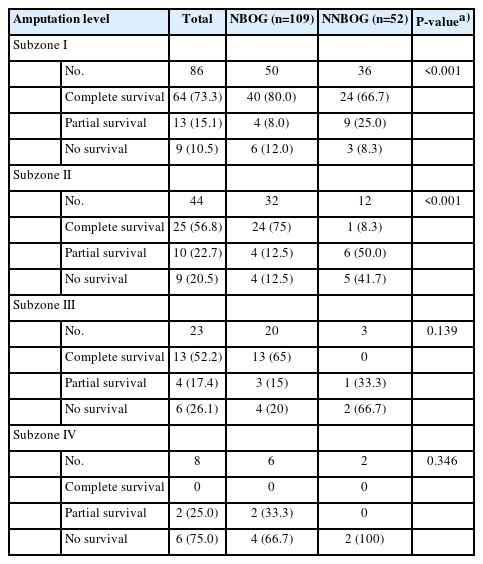
Graft survival was evaluated based on the amputation level. The complete survival rate was 73.3% in subzone I, 56.8% in subzone II, 52.2% in subzone III, and 0% in subzone IV. The effect of tNBO on the graft survival rate by amputation level was examined by performing a linear association analysis. For subzone I, grafts in NBOG showed a higher survival rate than those in NNBOG (80.0% vs. 66.7%, P<0.001). For subzone II, grafts in NBOG also showed a higher survival rate than those in NNBOG (75.0% vs. 8.3%, P<0.001). For subzone III, grafts in NBOG exhibited a higher survival rate than those in NNBOG (65.0% vs. 0%, P=0.139), although the difference was statistically insignificant. For subzone IV, there were no complete survival cases. In short, tNBO significantly increased the graft survival rate in subzones I and II (distal to the nail base area) (Table 3).
Composite graft survival risk factors
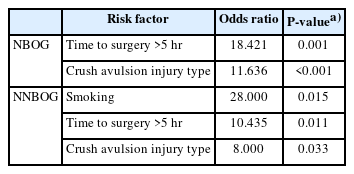
Five risk factors, namely, age >18 years old, smoking status, time to surgery (>5 hours), diabetes mellitus, and crush-avulsion-type injury, affecting composite graft survival [3,6,12,14, 15] were analyzed via binary logistic regression analysis. The reference group was defined as complete survival compared to partial survival and no survival. Time to surgery (odds ratio [OR], 48.6; P=0.001) and crush-avulsion-type injury (OR, 10.1; P<0.001) significantly decreased graft survival in both groups (Tables 4, 5). Smoking significantly decreased graft survival in NNBOG (OR, 28.0; P=0.015) but not in NBOG.
Discussion
This study was designed to evaluate the effect of tNBO on composite graft survival in fingertip amputation. This study had three major findings. First, tNBO increased composite graft survival. Without tNBO, the graft survival rate was 50%, which was similar to that reported in previous studies [8,9]. Graft survival increased by approximately 25% in tNBO-treated patients (75.3%). Araki et al. [19] also demonstrated that NBO increases composite graft survival in mouse models. Second, tNBO was effective in subzones I and II (distal to the nail base area). The diameter from the fingertip to the nail base is 1 cm or less in adults. HBO was also effective in composite grafts with a maximal diameter of 1 cm [23]. Complete survival was 0% in both groups in subzone IV. These results suggest that composite grafts should not be the primary choice in cases of fingertip amputation in subzone III or IV. Instead, replantation via microvascular anastomosis or other reconstruction methods may be considered. Third, tNBO decreased graft failure in smoking patients. Smoking is a well-known risk factor for graft survival due to cutaneous vasoconstriction and platelet aggregation [8,24]. Heistein and Cook [8] suggested that composite grafts should not be employed in smokers. HBO has been reported to decrease composite graft failure in smokers [25]. HBO increases the flap or graft survival rate in patients with a history of smoking, peripheral vascular disease, soft tissue infection, radiation therapy, or diabetes mellitus [17,25]. Similarly, in this study, smoking did not decrease the composite graft survival in NBOG, but increased graft failure by 28 times in NNBOG.
HBO and NBO can accelerate soluble oxygen levels in plasma [12,19,20], while HBO increases transcutaneous oxygen pressure 3 to 4 times that of NBO [12]. Nonetheless, in this study, tNBO substantially increased composite graft survival, which may be attributed to the direct administration of tNBO 24 hours a day. Continuous high oxygen levels in the injured area could contribute to an increase in composite graft survival.
Another advantage of tNBO is its simplicity and reasonable cost. A hyperbaric oxygen chamber costs more than US$10,000 and occupies a large space. In contrast, tNBO utilizes built-in wall oxygen flow systems normally used in hospital wards, with additional material costs of approximately US$10 and requires only limited space around the injured area.
This study had a few limitations. First, because the application of tNBO was determined by the surgeon’s choice and the patient’s consent, selection bias could have occurred during the process of selecting the treatment group. In some patients, tNBO was not administered because the patient refused to receive inpatient treatment or due to financial constraints. Second, we did not numerically quantify the effect of tNBO. The partial pressure of oxygen in arterial blood and the tissue partial pressure of oxygen in composite grafts with tNBO were not measured. Although the oxygen saturation of the tNBO-applied fingertip was measured as 100% by pulse oximeter in few patients, it only reflected the percentage of bound hemoglobin, and the exact amount of oxygen was not measured. Third, a standard assay of flow rate and tNBO duration was not performed; it was applied empirically. Fourth, a comparative analysis between HBO and tNBO was not performed. Therefore, further studies are warranted in these aspects.
In our study, tNBO improved composite graft survival in fingertip amputation distal to the nail base area (subzones I and II), but not proximal to the nail base area (subzones III and IV). tNBO decreased graft failure in smoking patients. Time to surgery (>5 hours) and crush-avulsion-type injury significantly decreased graft survival, regardless of tNBO. tNBO can be considered an acceptable adjuvant treatment in composite grafts in fingertip amputation distal to the nail base, especially in smokers.
Notes
No potential conflict of interest relevant to this article was reported.