Abdominal Wall Reconstruction in a Terminal-Stage Peritoneal Cancer Patient after Emergency Debulking Procedure: A Case Report
Article information
Abstract
Late-stage peritoneal cancer is a grave disease with a high morbidity rate; palliative care is the mainstay of treatment. In this article, we present a case of a terminal-stage cancer patient with sustained growth of the tumor. The mass was large enough to invade into the abdominal wall, and cause bowel obstruction as well as major vessel compression. From supportive care, we made a rapid pivot to emergent en bloc resection of the mass with a significant portion of the abdominal wall in one piece. After resection, a major part of the abdominal layers was deficient and required reconstruction. Due to rapid deterioration of the patient's condition, we changed our plan from autologous reconstruction to prosthesisbased reconstruction. Through this report, we share our decision-making process for reconstruction method selection, and practical considerations in the intraoperative setting.
Introduction
Development of multidisciplinary care and tumor boards has led to the use of multiple treatment modalities for the management of a single malignancy. This has resulted in more dynamic, yet comprehensive care for patients, with an increased survival rate and better quality of life throughout the course of treatment.
Terminal-stage cancer with intraperitoneal metastasis is usually considered unre-sectable [1]. Nevertheless, in certain cases involving invasion of the abdominal wall, removal of the tumor along with the abdominal wall and skin must be performed, after which immediate reconstruction is required. Therefore, the ability to add a reconstructive surgeon to the team is a critical hurdle in providing a surgical option to the patient.
In this report, we present a case of failed chemotherapy in a patient with terminal-stage cancer. The mass showed rapid expansion, inducing bowel obstruction and compression of large vessels, with invasion of the musculature and skin of the abdominal wall. The patient's condition showed rapid deterioration and the only apparent option was to proceed to hospice care. After an active discussion among multiple specialties, including the plastic surgery and general surgery departments, the patient underwent rescue debulking surgery with abdominal wall reconstruction (AWR). This study was approved by the Institutional Review Board of the Catholic University of Korea, College of Medicine (IRB No. KC22ZISI0373). The patient provided written informed consent for the publication and the use of her images.
Case
A 62-year-old female patient came into the emergency department complaining of abdominal pain, general weakness, poor oral intake, and fever. She had received low anterior resection for descending colon cancer 5 years prior. She also had a history of pulmonary thromboembolism, paroxysmal supraven-tricular tachycardia, and hyperthyroidism. She was admitted to the colorectal department for further evaluation of her condition. Abdominalpelvic computed tomography scans displayed multiple seeding metastasis of unconfirmed histology in the omentum, mesentery, pelvic cavity, and abdominal wall. A biopsy of the abdominal wall was undertaken to finalize the diagnosis.
Contrary to initial expectations, the biopsy revealed a different malignancy. A diagnosis of high-grade serous carcinoma was confirmed by immunohistochemistry for cytokeratin-7 (+) and cytokeratin-20 (–). Due to the multifocality and large extent of the tumor, the patient was not a candidate for resection. The patient was immediately transferred to the oncology department for further workup and preparation of chemotherapy to reduce the tumor burden and elongate her life expectancy by more than 6 months.
The patient underwent five rounds of paclitaxel and carboplatin chemotherapy over 4 months. Tumor response evaluation showed an increased size of the tumor with cystic degeneration (Fig. 1A). The tumor was invading about 40% of the abdominal wall musculature and skin (Fig. 1B). Due to the mass effect of the tumor, the patient developed severe ileus and hypotensive shock due to aorta compression. Switching to another chemotherapy regimen was impossible. The colorectal surgery and gynecology departments were consulted for the possibility of emergency debulking surgery. Since the tumor was invading through the abdominal wall, post-debulking re-construction was necessary for closure of the abdominal skin. Immediate reconstruction with plastic surgery was planned.
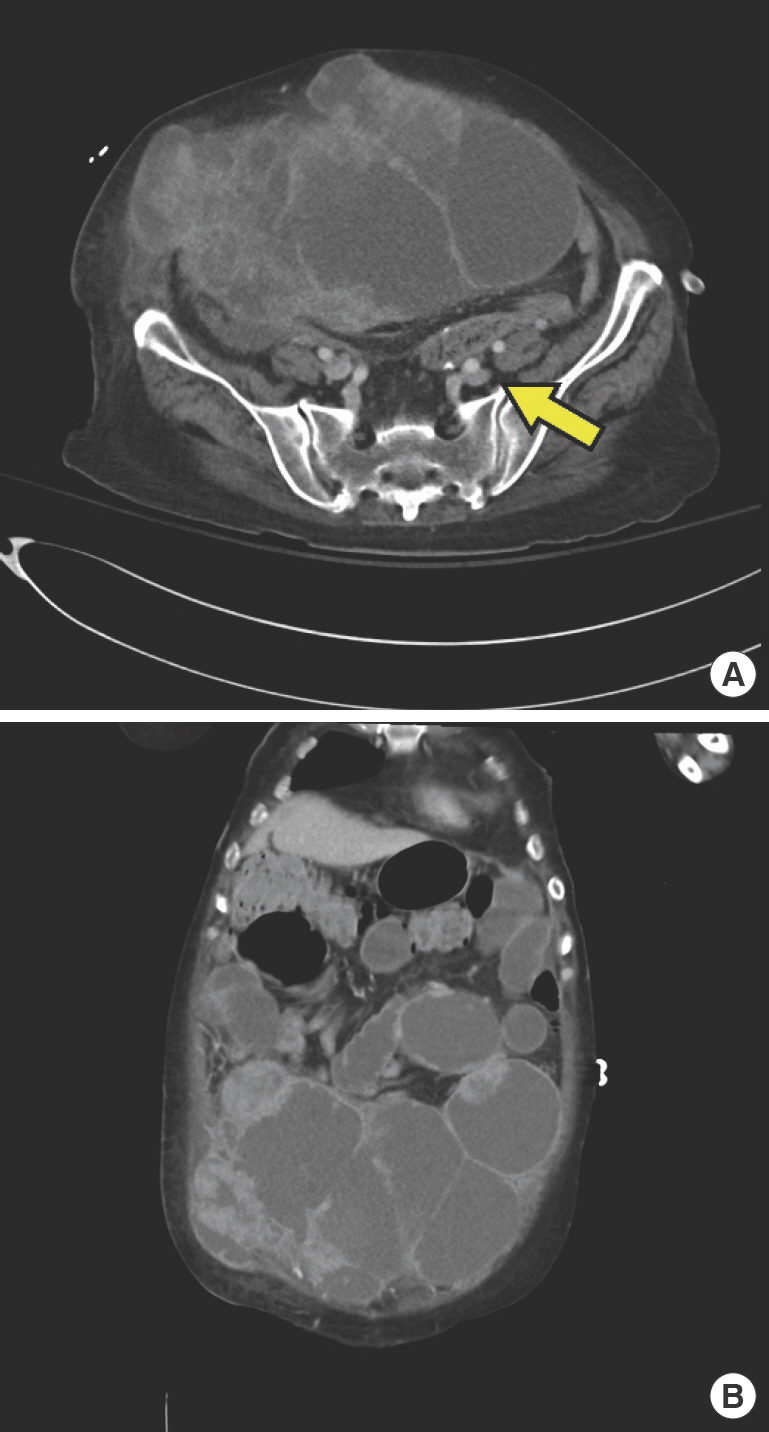
Preoperative abdominopelvic computed tomography of the patient. (A) Axial view. Tumor is invading into the abdominal muscle and soft tissues. Decreased diameter of the large arteries is noted due to mass effect (arrow). (B) Coronal view. The mass is involving about 40% of the abdominal wall musculature.
Colorectal surgeons performed en bloc resection of the small bowel and entire thickness of the abdominal wall including muscle, soft tissue and skin, and gynecologists performed a total abdominal hysterectomy with bilateral salphingo-oopho-rectomy. The size of the mass measured up to 25 cm in width, and weighed 1,079 g (Fig. 2). The total length of small bowel resection was unable to be defined due to severe adhesion of the tumor with the bowel. Approximately 150 cm of small bowel was left intact. After resection, plastic surgery was called in to reconstruct the resulting 30×35-cm-sized abdominal wall defect with bowel exposure. The prolonged procedure and massive intraoperative fluid resuscitation constituted a high risk of severe bowel edema and subsequent abdominal compartment syndrome. Therefore a staged reconstruction was planned, using a Silastic sheet (Reinforced Sheeting; Bioplexus Corp., Ventura, CA, USA) for provisional barrier formation and negative pressure wound therapy (NPWT) for the first stage.
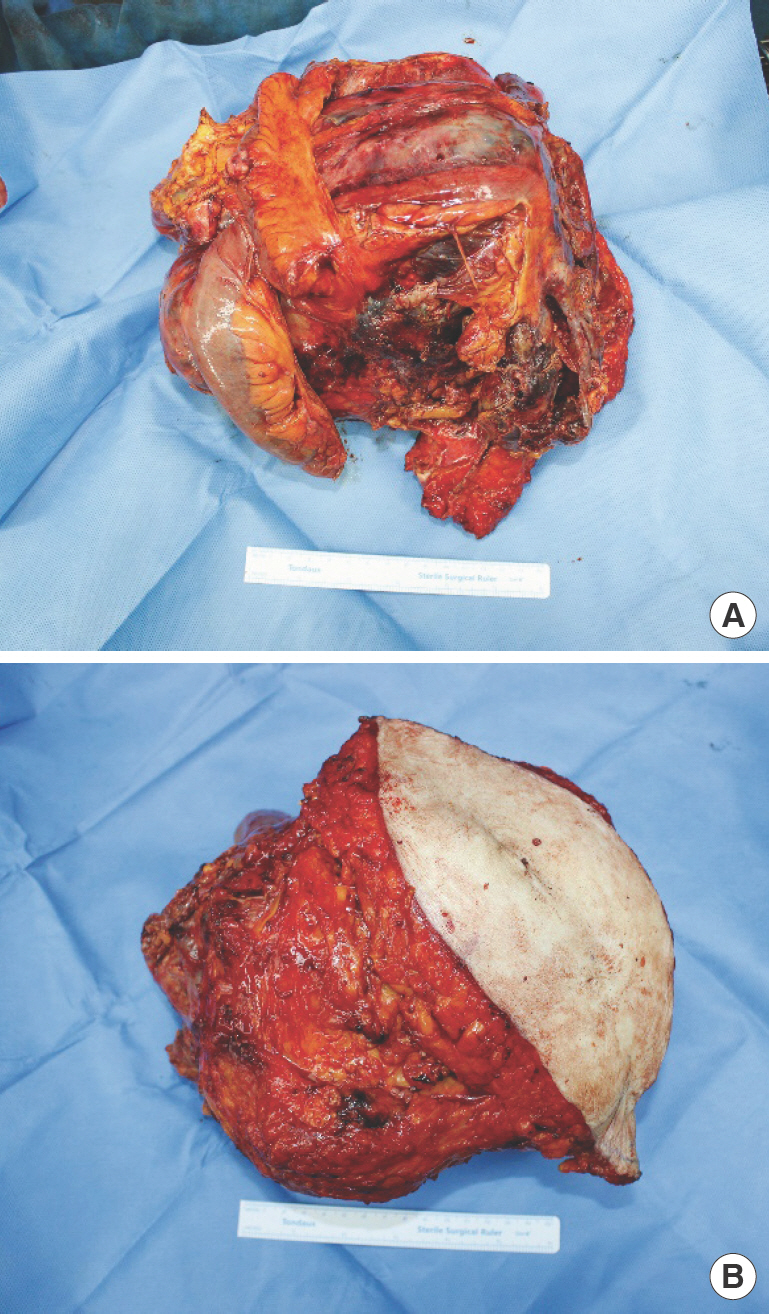
Gross photography of the tumor after resection. (A) External side. The mass was resected en bloc with skin and abdominal muscle. (B) Visceral side. The mass was measured to be over 20 cm.
A 25×15-cm-sized Silastic sheet was fashioned in a circular shape to cover the exposed bowel in the defect (Fig. 3A). Then, a NPWT polyurethane foam sponge (Curavac; CG Bio, Seoul, Korea) was applied into the defect to absorb serosal fluid and control bowel edema (Fig. 3B). An anterolateral thigh (ALT) flap was planned for a second-stage total AWR.
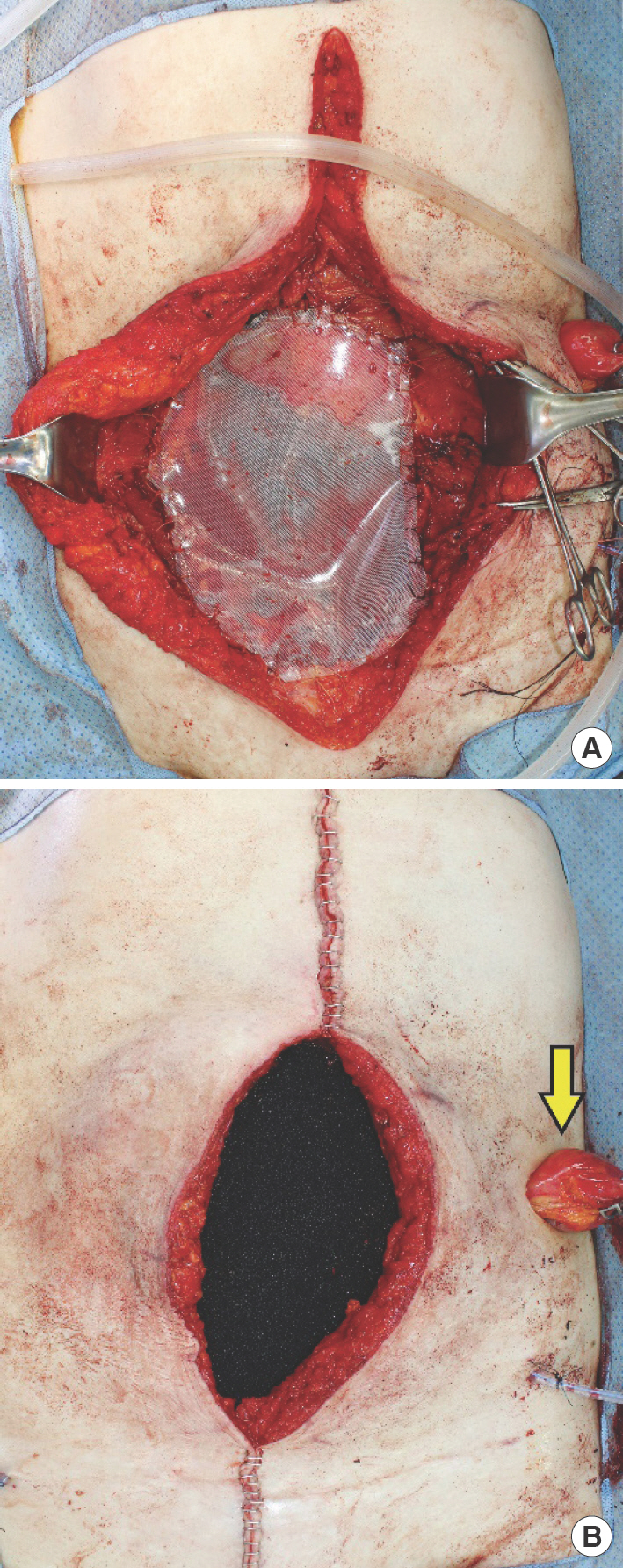
Intraoperative photographs of first stage reconstruction. (A) Application of Silastic sheet on the bowel and fixation around the margins of the fascial defect. (B) Interposition of negative pressure wound therapy sponge directly over the Silastic sheet. The ileostomy site is diverted far laterally to aid in easier airtight isolation of the bowel contents (arrow).
During 1 week of intensive care unit (ICU) care, the NPWT sponge was changed every 4 days under sedative anesthesia. While changing the NPWT sponge, the abdominal cavity was checked for signs of infection and bowel necrosis. On postoperative day 9, ischemic changes of the bowel and pelvic bleeding were discovered, and an additional portion (70 cm) of the small bowel had to be resected. Due to the prolonged period of ICU care and the patient's deteriorating general condition, flap reconstruction was deemed inappropriate, and plans were modified to attempt abdominal wall reconstitution using a bilaminar synthetic mesh (Symbotex; Covidien, Dublin, UK).
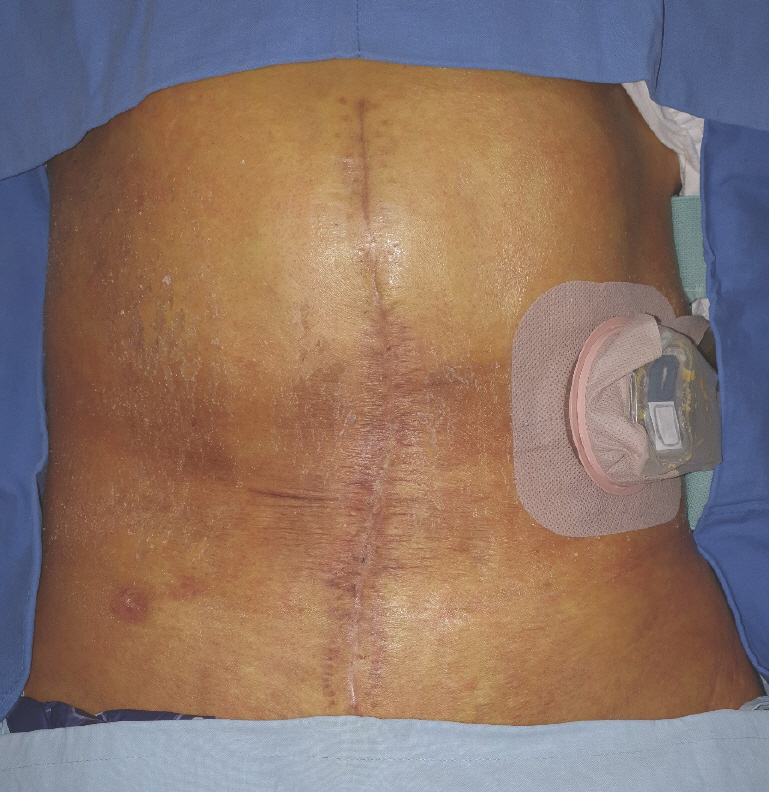
After 3 weeks' additional critical care, the patient's condition had improved sufficiently enough for general anesthesia, and the laparotomy wound was re-opened and explored toward the previously inserted Silastic sheet. Bilateral abdominal skin flaps were separated from the adhered Silastic sheet with meticulous handling. The sheet was maintained in a good state without infection signs. After locating the junctions of both edges of the abdominal wall and the Silastic sheet, the fixation sutures and sheet were removed, revealing intact bowel under-neath. The anatomical layer composed of the peritoneum, transverse abdominis (TA), and internal oblique muscle was identified. The peritoneum and internal oblique were each dissected from the TA, effectively developing two planes, one superficial and one deep to the TA. Due to the shortage of remaining abdominal musculature, partial coverage of the abdominal wall was achieved through bilateral release of the TA (Fig. 4A). After thorough irrigation, the bilaminar synthetic mesh was placed over the bowel and under the TA muscle in an underlay fashion (Fig. 4B). The mesh and bilateral margins of the released TA were sutured in a watertight manner (Fig. 4C), and drains were inserted in the abdominal cavity. Abdominal skin closure was possible without tension. On postoperative day 14, the drains were removed, and the surgical wound healed uneventfully. The patient is being followed up and seeking opportunities for additional chemotherapy for post-resection consolidation (Fig. 5).

Intraoperative photographs of second stage reconstruction. (A) Transverse abdominis release on both sides. The margin of one side is outlined (arrows). (B) Bilayered mesh application. (C) Schema of application of bilayered mesh combined with transversus abdominis release.
Discussion
As a result of advanced perioperative care and the development of surgical techniques, cancer patients once considered inoperable are now often selected as a candidate for surgery, leading to a dramatic reduction in oncologic burden and providing the patients a chance to receive additional adjuvant chemotherapy [2,3]. This case exemplifies such achievement in multidisciplinary care. Furthermore, the addition of reconstructive surgeons to the oncological management team further increases the opportunity for surgical management of patients with advanced status malignancies [4]. With aid from a reconstruction team who can provide restoration of the patient's form and function even after a morbidly ablative surgery, the oncologic surgeon can perform the resection with much more confidence and comfort.
High-grade serous carcinoma is notorious for its late presentation [1]. Therefore, the majority of patients are seen in an advanced state, which precludes surgical management as a primary treatment option. Our case was similar where surgery as a treatment option was not possible due to the widespread nature of the tumor. Unfortunately, tumor growth was not suppressed even after multiple rounds of chemotherapy, and pathological mass effects began to occur on the gastrointestinal tract and major vessels. In this situation, rescue debulking surgery was the only option to alleviate the mass effect and overcome the patient's critical deterioration. When planning an extensive resection, as in our case, the availability of a reconstructive surgeon, rather than the resection itself, is often the most important precondition of the entire decision-making process.
AWR can be achieved with autologous tissue, or prosthetic devices such as synthetic mesh and acellular dermal matrix. For a full-thickness defect of the abdominal wall where fascial primary closure is impossible, the use of a composite flap including muscle and fascia is generally necessary in order to recreate the layered anatomy of the abdominal wall. For this reason, autologous reconstruction is the first line option for total AWR, with the ALT flap regarded as a workhorse flap [5]. The ALT flap can be utilized in various sizes and combinations of tissue types such as muscleonly, fasciocutaneous, or mus-culofasciocutaneous composites. In cases where microsurgery is contraindicated, transfer of the flap can be performed in a pedicled fashion [6]. If patient-specific factors preclude the use of the ALT flap, a vertical rectus abdominis musculocutaneous flap or local flap with fascia lata can be an alternative option. However, these can only be used when the defect size is significantly small enough to permit re-arrangement of the local abdominal tissue.
If primary closure of the fascia and skin can be achieved, prosthetic reconstruction with mesh or dermal matrix is a feasible option. Since our patient lost all layers of her abdominal wall including skin, fascia and muscle, an ALT flap was initially planned. However, due to the patient's critically deteriorating condition, our plan was changed to insertion of a mesh with TA release, which enabled a shorter surgical procedure and easier postoperative recovery.
For complex AWR, the possibility of a concomitant, single-stage AWR is usually the first option in the reconstructive surgeon's thought process. However, in our case, single-stage re-construction was not indicated due to the high risk of severe bowel edema. According to the algorithm suggested by Petro and Rosen [7], concomitant AWR should be deferred until he-modynamic stability is achieved and bowel edema is resolved. Therefore, NPWT with a Silastic sheet was implemented as a bridging therapy while waiting for resolution of the bowel edema. A Silastic sheet was applied directly to the bowel with interposition of a NPWT sponge between the abdominal defect and the Silastic sheet. Use of this method resulted in isolation of the open abdomen from the external environment and the bowel edema was cleared out effectively. Placing the ileostomy/colostomy site as far lateral as possible aids in maintaining NPWT without air leakage, enabling unhindered application of the airtight film dressing over the NPWT sponge.
AWR with mesh insertion is a common practice in management of benign conditions such as incisional hernia. Single-stage AWR with fascial reinforcement and mesh implantation is gaining popularity due to the development of sophisticated abdominal wall release techniques and the advent of infection-resilient mesh barriers [8]. However, in cases involving complex reconstruction such as our patient, it is not a common practice to implement mesh-only interposition in a bridging fashion into fascial gap where primary fascial closure is impossible [9]. In our case, this was enabled by the unique features of the next generation bilayered mesh product. The product is a bilayered construct composed of nonabsorbable polyester monofilament and a bioabsorbable collagen film. The absorbable collagen side enables direct application to the bowel and subsequently promotes neoperitonization through mesothelial cell migration along the bowel contact surface [3]. On the outer surface, the clinging effect of the nonabsorbable polyester mesh to abdominal soft tissue results in broad adhesion, preventing bowel hernia [9]. In our opinion, these distinctive features give the bilayered mesh product a major advantage over monolayer mesh or acellular dermal matrix when the prosthesis is implanted in a bridging manner. With the expectation of weaker abdominal wall strength compared to use of a conventional onlay technique with primary fascial closure, the patient was taught to wear an abdominal binder for a longer duration in order to facilitate adequate integration of tissue around the mesh. Another important issue is the method used for fixating the mesh, and in which layer the mesh will be interposed [10]. Because the mesh was interposed as a single layer without autologous fascia, the TA was released as widely as possible and the mesh was placed in an underlay fashion.
We present an atypical case where surgery was not initially indicated. Based on our experience, we hereby suggest an algorithm to aid decision making regarding AWR methods (Fig. 6). The importance of the reconstructive team cannot be overem-phasized when planning for complete resection. In cases where fascial primary closure is impossible but flap reconstruction is contraindicated, use of a bilayered mesh can be an adjunct to promote neoperitonization on the inner surface, and tissue integration on the extraperitoneal surface. Furthermore, TA release can reduce fascial defect size and help achieve underlay fixation of the bilayered mesh.
Conflict of interest
No potential conflict of interest relevant to this article was reported.