Successful Perforator-Based Island Flap Placement Using the Push Technique
Article information
Abstract
Background
Several surgical options are available for covering soft tissue defects. Perforator-based flaps can be utilized without skeletonization of the perforator, and surgery is usually straightforward, albeit with limited arcs of rotation. To overcome this limitation, we present our experience of the push technique, whereby the flap is manually pushed to release soft tissue attachments, allowing circumvention of the meticulous dissection required to enhance arc rotation. This technique allows faster flap elevation with minimal complications.
Methods
The records of 37 patients that underwent perforator-based flap surgery using the push technique from September 2020 to June 2021 at our institution were retrospectively reviewed.
Results
All 38 flaps survived completely though one flap developed congestion resulting in partial necrosis and revision. Mean operation time was less than 80 minutes. No dehiscence or recurrence was encountered at any donor or recipient site during follow-up.
Conclusion
We present the “push technique” used during perforator-based flap surgery. This method allows for efficient, minimal, and rapid flap elevation without meticulous dissection and helps the operator determine which parts of soft tissue attachments require further release. The push technique can be performed safely and easily without jeopardizing flap survival due to venous ischemic or congestive change. It serves as a good practice model for inexperienced surgeons wishing to cover moderateto large-sized defects.
Introduction
Flap surgery is a fundamental reconstruction method for covering defects. Many surgical methods have been proposed, and perforator flaps are commonly used world-wide [1–5]. Perforator flaps are popular because they allow reconstruction of wide defects requiring long pedicles and large rotating arcs while preserving underlying muscles. However, meticulous perforator dissection is required, and for less experienced surgeons, skeletonization requires much effort [6–10]. In addition, pedicle twisting or postoperative compression can cause venous ischemia and congestion.
Perforator-based island flaps (PBIF), with the perforator located near the defect and flap elevation halted around the perforator, have been used to avoid fastidious dissection and vascular compromise. However, leaving 1–2 cm of soft tissue around the perforator limits arcs of rotation to around 60°. Furthermore, as PBIF have more soft tissue attachments around the perforator compared to true perforator flaps, flap twisting around the pivot point may occur when the flap is rotated. Additional measures are required to rotate flaps by 90° [11]. In this study, we present our experiences of PBIF surgery using the “push technique” to release soft tissue attachments. This technique does not require meticulous dissection to enhance arcs of rotation and is performed by manually pushing flaps toward the defects.
Methods
The records of patients who underwent perforator-based flap surgery using the push technique at our institution from September 2020 to June 2021 were retrospectively reviewed for patient age, sex, etiology, defective location, defect size, flap size, operation time, complications, and follow-up times. Thirty-eight PBIF procedures were carried out on 37 patients (22 men and 15 women) of the mean age of 59.1 years (range, 33–94 years). Defects were localized at the sacrum (28.9%), greater trochanter (23.7%), ischium (15.8%), back (13.2%), thigh (5.3%), scrotum (5.3%), anus (2.6%), vulva (2.6%), and coccyx (2.6%). Twenty-five flaps were required for pressure sores, 12 for wounds resulting from cancer excision, and one for treating infection. Average flap size was 135.8 cm2 (range, 3×4 to 17×12 cm). Table 1 summarizes relevant patient information.
This study was approved beforehand by the Institutional Review Board of Chungnam National University Hospital (IRB No. CNUH 2021-05-042) and performed in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent for publication and use of their images.
Operative techniques
Locations of perforators around defects are determined after debridement or excision using a handheld acoustic Doppler probe. Perforators adjacent to defects are selected, and elliptical flap designs are used for primary defect closure. When donor sites are pinched to simulate actual closure, defect shapes become narrower and longer, and thus, flap designs are modified accordingly. Flaps are designed to be 1–2 cm longer than the actual defect lengths (from pivot points to the margins of recipient defects) (Fig. 1).
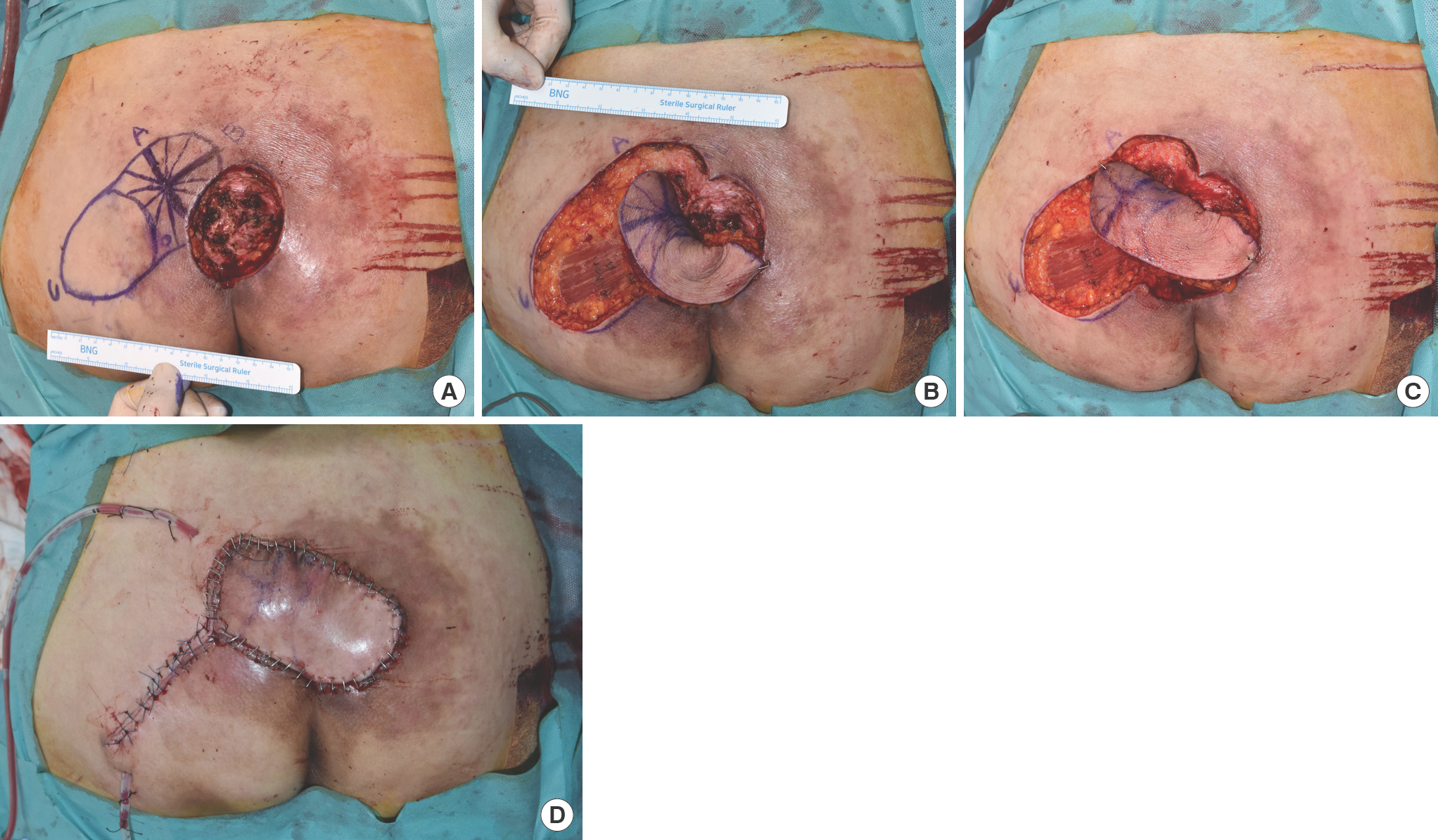
A 63-year-old quadriplegic woman with a sacral sore. (A) Design of the parasacral perforator-based island flap (defect size 6×7 cm, flap size 14×7 cm). (B) Notice twisting around the pivot point before the push technique was applied. (C) The flap was transposed without any tension or twisting after applying the push technique. (D) The elevated flap was sutured in layers.
Flaps are elevated distally toward the pivot point, usually in the subfascial plane, but may include some muscle if necessary. Dissection is halted when the flap tip can be transposed to the defect. It is important to maintain a distance of at least 1 to 2 cm from the perforator because soft tissues around perforators prevent perforator twisting, kinking, or compression during flap transposition and after surgery. However, leaving soft tissue around a pivot point limits flap rotation and may result in flap twisting around the pivot point, possibly causing flap ischemia and congestion.
To overcome this limitation, we push the flap manually and firmly towards the defect to release soft tissue attachments (Supplementary Video 1). Sometimes tearing of soft tissue is sensed between muscle fascia and deep fat. After this procedure, most flaps are transferred to defects without tension or flap twisting around the pivot point. However, if flap twisting occurs after applying the push technique, minimal additional incisions are made as needed to the muscle fascia or muscle while pushing the flap. No meticulous dissection is performed around perforators, saving time and increasing the likelihood of flap survival. Elevated flaps are transferred to defects and sutured in layers. For flap widths of <8 cm, the donor site is usually closed primarily without undermining, while for flap widths >8 cm, both sides of the donor site are undermined for primary closure. No pressure should be applied on the flap until 1 week postoperatively, after which pressure application of within 1 hour at a time is permitted up to 3 weeks, and then no such pressure restrictions after the 3rd week. Patient posture varies depending on the surgical site. In the case of sacral pressure sores, the patients are allowed to lie down from 3 weeks after surgery and to sit down from 6 weeks. If the surgical site is located on the back, patients are instructed to refrain from lying down supine on the flap for about 3 weeks including while sleeping, after which no other restrictions are necessary. With anterior surgical sites, only a postoperative bed rest period of 2–3 days is required. Unless the surgical site is in a weight-bearing region, postoperative day 3 ambulation is recommended for ambulatory individuals.
Results
Flaps with dimensions of 4×8 cm to 25×9 cm were elevated, and 37 of the 38 flaps survived completely. One patient developed flap congestion resulting in partial necrosis requiring another flap (case 2). However, no dehiscence or recurrence was encountered during follow-up (range, 2–9 months; mean, 4.9 months). Mean operation time (from completion of unhealthy tissue or cancer excision to closure of wound) was 79.6 minutes. Below we present four clinical cases in which the push technique was successfully used (cases 1, 2, 3, and 4).
Case 1
A 63-year-old quadriplegic woman presented with a sacral pressure ulcer that had persisted for 6 months. After radical debridement, a 6×7-cm-sized defect remained. Perforator locations around the defect were determined preoperatively using a handheld acoustic Doppler probe, and a 14×7-cm-sized fasciocutaneous flap was designed as described above (Fig. 1). The flap was elevated, and dissection was stopped 2 cm from the designated perforator. However, when the flap was transposed to the defect, soft tissue attachments around the pivot point limited flap rotation and caused flap twisting. Pushing the flap toward the defect released the soft tissue attachments between muscle fascia and deep fat, and the flap was comfortably transposed to the defect without any tension or twisting. The elevated flap was sutured in layers, and donor wound closure was performed primarily. No wound dehiscence or ulcer recurrence occurred, and soft tissue remained stable at her 4-month follow-up.
Case 2
A 59-year-old paraplegic man presented with a greater trochanter pressure ulcer that had persisted for 2 months. Radical debridement created a 7×8-cm-sized defect. A 16×8-cm-sized flap (tensor fasciae latae PBIF) with a stellate design [12] was elevated and the push technique was used as described above (Fig. 2). In this case, the A and B lobes of the flap were designed to ease donor site closure but could not be located in the planned position due to an incorrect design. As a result, the flap was exposed to excessive tension, which led to congestion. Accordingly, most of the sutures around the flap were removed except for 2–3 staples. For 5 days after surgery, a local subcutaneous injection of low-molecular-weight heparin was administered, and the flap was rubbed with heparin gauze every 2 hours. Nonetheless, necrosis of the flap margin progressed, and as a result, only about 40% of the flap survived.
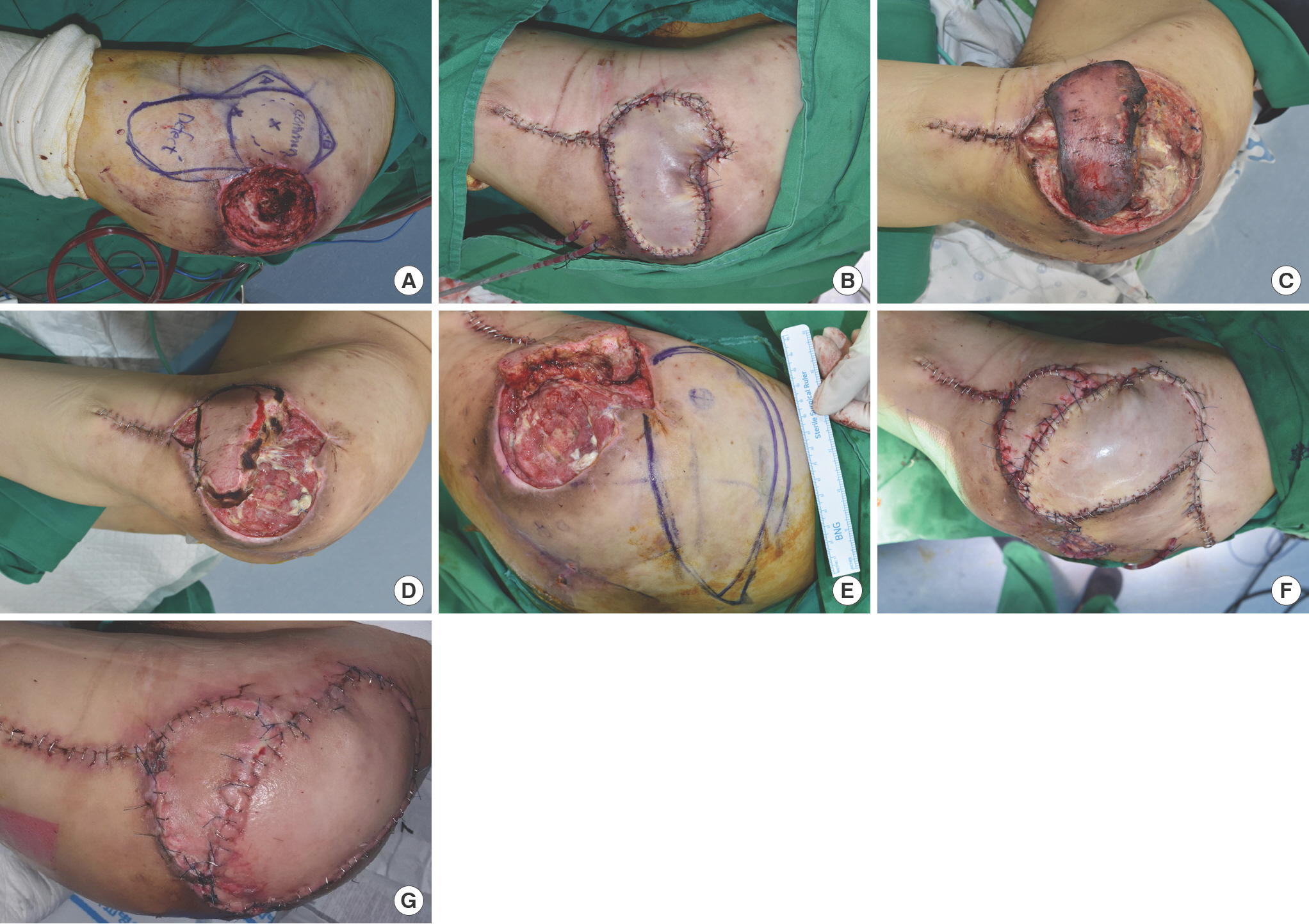
A 59-year-old paraplegic man with a greater trochanter sore. (A) Design of the tensor fasciae latae perforator-based island flap (defect size 7×8 cm, flap size 16×8 cm). (B) The flap was transposed using the push technique. The elevated flap was sutured in layers. (C) Flap congestion was observed, and most of the sutures around the flap were removed except for 2–3 staples at 2 days after surgery. The picture shown was taken on the 5th postoperative day, and although congestion had improved, marginal necrosis had progressed. (D) At 2 weeks postoperatively, about 40% of the flap remained, and (E) another tensor fasciae latae perforator-based island flap was transposed (F) and sutured in layers. (G) No dehiscence or complication was observed during the 2 weeks following the second procedure.
Two weeks after surgery, the defect could not be covered with the remaining flap; another tensor fasciae latae PBIF using a proximal perforator was used. A back cut was performed for donor site closure, and additional skin grafts (3×4 cm) were required. Subsequently, the wound remained stable and no additional wound dehiscence occurred.
Case 3
A 47-year-old man presented with a multifocal basal cell carcinoma on his back, which was removed by Mohs micrographic surgery at our dermatology department. Excision created a 7×8-cm-sized skin defect (Fig. 3), and a 16×8-cm-sized fasciocutaneous flap was designed as described above. The flap was elevated, and the push technique was used. Three months after surgery, there was no wound dehiscence, and soft tissue remained stable.
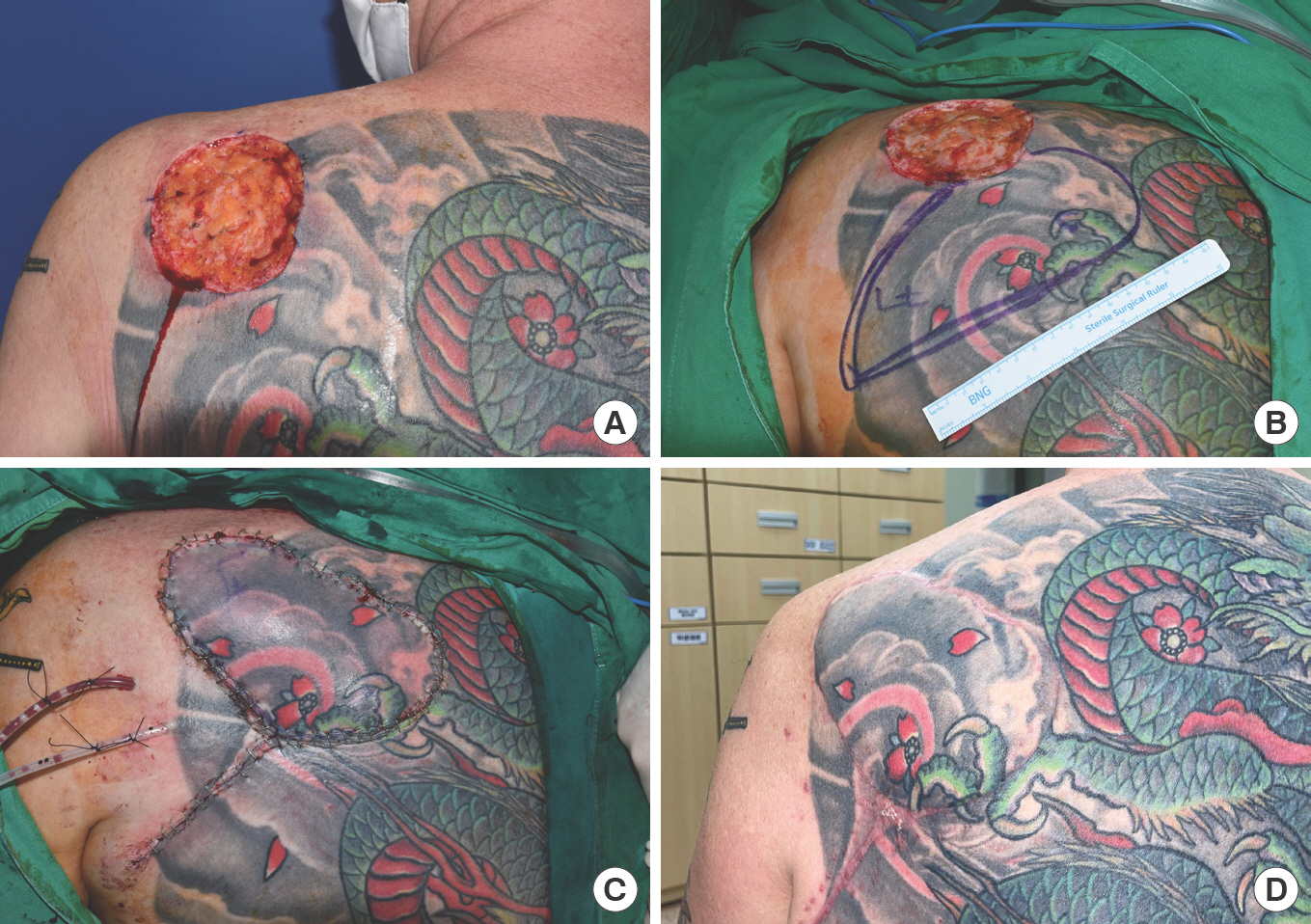
A 47-year-old man with a back defect. (A) Skin defect left after multifocal basal cell carcinoma excision. (B) Design of the dorsal scapular artery perforator-based island flap (defect size 7×8 cm, flap size 16×8 cm). (C) The flap was transposed using the push technique without tension or twisting and sutured in layers. (D) Photograph of the flap at 3 months postoperatively.
Case 4
A 75-year-old man with cerebral infarction and diabetes history had a chronic ulcer on his back that had persisted for 6 months and was referred to our hospital. The ulcer started as a small, unhealed wound and was positive for Pseudomonas ae-ruginosa. Primary repair and rotational flap placement were performed at another hospital, but the wound did not improve. At our hospital, radical debridement created an 8×6-cm-sized defect. A 16×6-cm flap was designed, and the push technique was used as described above (Fig. 4). No wound dehiscence occurred, and soft tissue remained stable at his 2-month follow-up.

A 75-year-old man with a back defect. (A) The skin defect was caused by an unhealed chronic ulcer. (B) Design of the thoracodorsal artery perforator-based island flap (defect size 8×6 cm flap size 16×6 cm). (C) The flap was transposed without flap tension or twisting using the push technique and sutured in layers. (D) Photograph of the flap at 2 months postoperatively.
Discussion
Various surgical options have been used to cover medium to moderate-sized defects, but all have their merits and demerits. The introduction of the perforator flap has revolutionized reconstructive surgery over the past 20 years [5]. This flap technique minimizes donor site morbidity and allows extensive defect reconstruction using a long pedicle and a thin flap without underlying muscle. However, long pedicle harvesting with source vessel isolation increases the risk of vessel injury leading to venous congestion or ischemia and possibly flap survival failure.
The perforator-based flap can overcome the disadvantages of the perforator flap. In the case of perforator-based flaps, flap elevation is stopped at the level of the perforators. As a result, the time and effort required for perforator skeletonization and intramuscular dissection can be reduced [2]. Although perforator-based flaps can reduce complications and operation times, tension at donor sites is greater than that caused by perforator flaps. Kim et al. [11] stated that their PBIF and tension-free closure technique could be achieved by perfecting flap design, which usually involved the use of a flap longer and narrower than defect dimensions to facilitate primary donor site closure. Initially, we followed their PBIF elevation technique (distal to proximal elevation while leaving about 2 cm of soft tissue around the perforator), but flap elevation alone proved insufficient to allow a flap rotation of 90°. To achieve this level of rotation after flap elevation and transposition, we pushed flaps manually and firmly toward defects to release soft tissue attachments. Using this technique, flaps were transposed comfortably without tension or twisting. When a flap exhibited tension after the push technique, we were able to easily identify the part of the flap under tension while pushing the flap, and additional minimal incisions were made on the muscle fascia or the muscle to release tension. Accordingly, our method precludes meticulous dissection of perforators, which saves time without jeopardizing flap survival.
Because the perforators were not skeletonized and not visually inspected, the exact condition of the perforators could not be determined after the maneuver. However, we have not experienced flap loss due to perforator damage, and we consider that the push technique separates soft tissue around perforators rather than directly affecting the perforators themselves.
In addition, when a perforator flap is applied to a mobile area, such as the greater trochanter or back region, congestion or ischemia may occur after surgery due to excessive tension caused by changes in posture or exercising after surgery. However, leaving a certain amount of soft tissue around the perforator dissipates tension applied to the perforator and reduces complications.
The advantages of our method are that it can be performed easily and safely, and thus, may be suitable for young surgeons with limited experience of perforator isolation. On the other hand, it requires some experience to control the force applied during pushing so as not to detach the whole flap, and considerable experience of flap design to achieve tension-free closure. Furthermore, the described method can be used when the arc of rotation required is around 90°, such as in our cases. However, when an arc of rotation of more than 90° is required, a perforator flap with skeletonization should be considered.
The method presented in this study is considered suitable for areas such as the back, thigh, and buttocks which have multiple perforators available around defects and redundant soft tissue to facilitate donor site primary closure. In most cases, flap rotation of up to 90° is possible. On the other hand, the authors do not consider the push technique suitable for lower leg reconstruction because perforators are often far removed from defects and/or require rotation of >90°, which means perforator skeletonization is required in many of these cases. Furthermore, most of the donor sites require a skin graft because primary closure is not possible.
As with other local flaps, flap design is critical when our method is used, as incorrect design can result in flap tension and cause congestion (case 2). If donor site closure is difficult or requires flap rotation of >90°, it is possible to cover the defect and the donor site at the same time by designing the flap slightly longer than usual and bending the flap itself.
The limitations of the present study are that the sample size was relatively small and follow-up times were short. Furthermore, statistically meaningful comparisons with other studies were not possible because flap locations and defect sizes differed. Nonetheless, the results obtained are promising. Sameem et al. [13] reviewed 55 previous studies related to pressure sores and reported a pressure ulcer recurrence rate of 5.6% and complication rate of 19.6% including wound dehiscence, infection, and necrosis in perforator-based flaps. Bamba et al. [14] reviewed 276 patients who underwent flap coverage of pressure ulcers, and reported a recurrence rate of 28.6% and a complication rate of 31.2%. In our study, when using the push technique, the relapse rate was 0% and complication rate was 2.63%. Though admittedly with a short follow-up period, we had no cases of wound dehiscence or recurrence, and congestion (2.63%) occurred in only one patient, suggesting that it is a safe method. Although the push technique is not suitable for all cases, in our opinion, it provides an easier alternative for inexperienced surgeons.
Conflict of interest
This work was supported by research fund of Chungnam National University Hospital. This work was supported by Chungnam National University Hospital Research Fund, 2021. This
work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2021R1G1A101369411).
Supplementary material
Supplementary Video 1.
Video of the “push technique”. Using gauze, the flap was pushed in the direction of the defect to separate the attachments between muscle fascia and deep fat tissue. Notice the reduction in twisting achieved after applying the push technique.
Supplemental data can be found at: https://doi.org/10.22467/jwmr.2021.01893.

