Introduction
Negative-pressure wound therapy (NPWT) is widely used for a range of acute and chronic wounds [1]. NPWT helps wound healing by maintaining moisture, removing exudate, increasing local vascularity, decreasing bacterial colonization, stimulating granulation tissue, and so on [2].
However, it is contraindicated in cases with malignancy, necrosis, fibrosis, exposed vessels, and uncontrolled infection [3]. Moreover, indications of NPWT have been varied, whereas contraindications of NPWT are still controversial [4]. For this reason, deciding on a therapeutic option is often challenging in cases of contraindications.
We hereby report our experience with a case where NPWT was used to treat a replanted finger with exposed blood vessels and severe infection, which are contraindications to NPWT, and discuss precautions for such treatment.
The study was approved by the Institutional Review Board of Gwangmyeong Sungae Hospital (IRB No. KIRB-2020-N-005) and performed in accordance with the principles of the Declaration of Helsinki. The patient provided written informed consent for the publication and the use of his images.
Case
A 60-year-old man with amputated fingers after an injury caused by a press machine visited our emergency department. We immediately performed replantation of his left index and middle fingers (Fig. 1A). Whereas both digital arteries of the amputated index finger could be directly anastomosed, the ulnar and radial digital arteries of his middle finger could be anastomosed only with vein grafts, owing to thrombi of both digital arteries (Fig. 1B and C). Although the patient’s two replanted fingers survived, necrosis and methicillin-resistant Staphylococcus aureus (MRSA) infection in the middle finger was noted through wound culture on postoperative day 10. The complex wound showed little improvement even after 2 weeks of intravenous injection of vancomycin (1 g twice a day). Due to his active infection, the patient was not in a state to receive another flap surgery to cover the wound. Since previous replantation had been done with vein grafts for both digital arteries of the middle finger, the finger could not provide stable arterial blood stream for additional flap coverage.
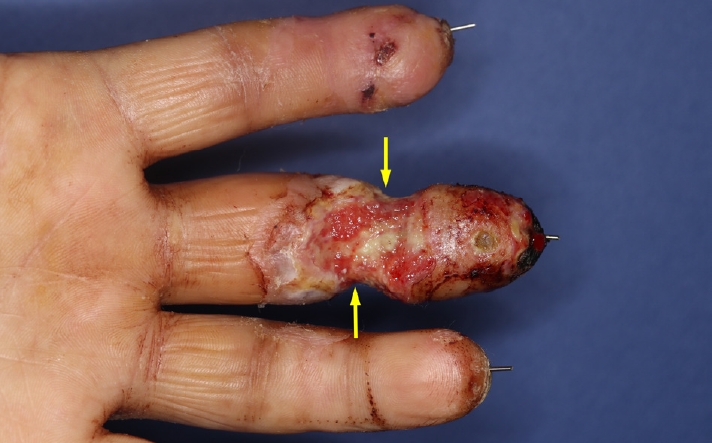
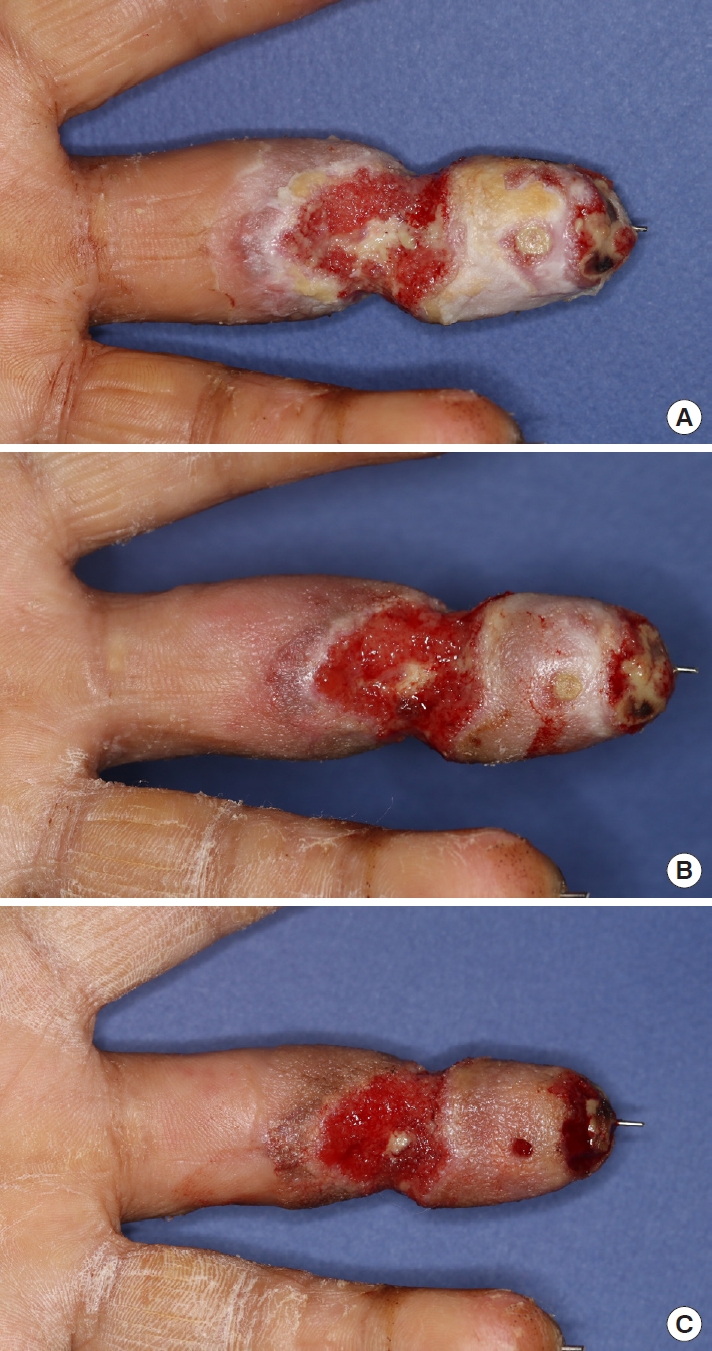
Therefore, even though the wound was intractable to infection control measures (Fig. 2), we decided to conserve the replanted finger and apply negative pressure of –75 mmHg at an intermittent mode (5-minute suction and 2-minute rest) to prevent ischemic change, for the first 3-day cycle of NPWT, using an InfoV.A.C. Therapy Unit (KCI Medical, Inc., Mississauga, ON, Canada) after debridement. The wound was sealed with a NPWT sponge, opened laterally to minimize the exposure of the vessels (Fig. 3A). The blood circulation in the replanted finger was maintained for the first cycle, and granulation tissue began to form and cover both pedicles (Fig. 4A). We increased the negative pressure up to –100 mmHg for the second cycle, keeping the same intermittent mode and sealing technique with the sponge opened laterally, as in the previous cycle (Table 1, Fig. 4B). In the third cycle, we sealed the finger circumferentially since both vessels were fully covered (Table 1, Fig. 3B). After three cycles of NPWT, the granulation tissue fully covered the exposed digital vessels (Fig. 4C) and signs of infection such as erythema, odor, and discharge decreased. Inflammatory markers such as erythrocyte sedimentation rate and C-reactive protein level decreased from 45 mm/hr and 3.82 mg/dL respectively on postoperative day 10, to their normal ranges on postoperative day 27. On postoperative day 43, the final follow-up wound culture confirmed the absence of bacterial growth. Finally, we covered the wound with only a split-thickness graft and discharged the patient on postoperative day 66.
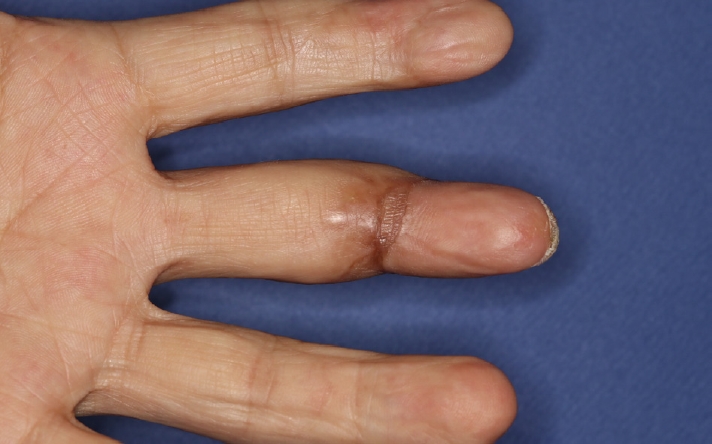
The patient’s replanted left middle finger survived well without any circulatory complications and maintained full coverage and intact blood circulation, as confirmed in tracings with a handheld Doppler ultrasonographic device at 2 and 4 months after discharge (Fig. 5). Our primary goal was to cover the wound as soon as possible, and the contracture caused by split-thickness skin graft on distal phalangeal joint had been expected. Indeed, this patient had tenolysis surgery of the finger and improved range of motion of the replanted finger 3 years after discharge (Fig. 6).
Discussion
NPWT is widely known to be useful for managing various types of wounds at different anatomical sites. It has been recently modified with a variety of applications and methods [1]. It is thought to help promote wound healing through several mechanisms such as macrodeformation, microdeformation, fluid removal, and optimization of the wound environment. NPWT thus plays a significant role in managing chronic, complex, and infected wounds [1,2].
Although some secondary procedures after replantation have been studied, the use of NPWT in such cases has not yet been reported [5,6]. NPWT is also not recommended for managing wounds with MRSA infection [7].
In this case, application of NPWT for a complex wound with exposed vessels and uncontrolled infection had been disputable. However, the condition of the replanted finger was not suitable for coverage with a free flap and vein graft or a regional flap, because the adjacent index and ring fingers were crushed. In addition, without immediate coverage, all portions of the replanted finger would have had necrosis.
Although no cases using NPWT to treat wounds of replanted fingers have been reported, we nevertheless considered NPWT as a means to conserve the replantation, and needed an appropriate modulation for applying NPWT to the complex wound. To maintain blood flow, the negative pressure applied was not to exceed the average finger blood pressure (110 mmHg), and the NPWT sponge was to be used for sealing only on the volar and dorsal sides of the wound in the first NPWT cycle, to prevent choking the wound with exposed vessels [8]. The sponge could then be sealed circumferentially in the second and third cycles, after checking that granulation tissue had covered the wound pedicles, and using a handheld Doppler ultrasonographic device to confirm the blood flow was intact.
In general, the negative pressure applied in NPWT ranges from –125 to –175 mmHg, in intermittent or continuous mode. A negative pressure of –125 mmHg is the baseline setting for all kinds of wounds. The effect of the intermittent mode (5-minute suction and 2-minute rest) on wound healing is equivalent to that of the continuous mode [9]. Moreover, as granulation tissue formation takes place in the intermittent mode as much as in continuous NPWT mode, the NPWT mode should be tailored to individual wounds for optimal effects [10]. According to Wackenfors et al. [11], the effective negative pressures for minimizing possible ischemic effects were –75 mmHg for soft tissue and –100 mmHg for muscle tissue. Accordingly, we applied a –75 mmHg pressure for the first cycle and increased it to –100 mmHg for the second and third cycles, maintaining the intermittent mode.
Even though NPWT is being applied for an increasingly broader range of wounds, anatomical sites, and medical conditions, whether the replanted finger in this case can be considered a definite indication for NPWT might not be affirmed. Nevertheless, we demonstrated that a wound with vascular exposure and active MRSA infection could be safely managed with NPWT. We therefore conclude that NPWT could be a possible option for replantation with intractable wounds under appropriate negative pressure modulations.