Introduction
Extracorporeal shock wave therapy (ESWT) is a standard treatment for urinary stones. In orthopedics, specifically in Japan, ESWT is covered by insurance only for refractory plantar fasciitis. Meanwhile, the Consensus Statement of the International Society for Medical Shockwave Treatment (ISMST) [1] has indicated that ESWT is a feasible and noninvasive treatment for intractable skin wounds and skin ulcers; these findings are congruent with those of a previous systematic review [2] and meta-analysis [3]. Some recent studies have examined ESWT for hypertrophic and postburn scars [4].
In refractory skin wounds, the peripheral limbs are prone to decreases in blood flow due to peripheral circulatory insufficiency, and wound healing is likely to be delayed owing to the lack of soft tissue. In addition, motor functional structures, such as tendons and bones, are susceptible to infection and are therefore prone to becoming dysfunctional [5].
The Bates-Jensen Wound Assessment Tool, proposed by Bates-Jensen et al. [6,7], and DESIGN-R scoring manual, advocated by the Japanese Society of Pressure Ulcers [8], are designed to assess wound status and monitor healing. However, when these tools are used to assess the periphery of the extremities, it is difficult to ascertain a difference in the scores regarding wound size or edges. Further, it is challenging to compare the progression of wound healing before and after treatment. In this study, we used our newly developed assessment tool (Hand and Foot Wound Assessment Tool, HFWAT) to evaluate intractable skin wounds on the hands and feet to determine the utility of ESWT for skin wounds with delayed healing.
Methods
Patients
This study was approved by the Ethics Committee of Murakami Surgical Hospital (approval number: M50801) and conducted by two physicians (TS and HM) and one physical therapist (KK). Seven patients with persistent skin wounds that did not display signs of healing after undergoing usual treatments for 2 weeks or more who visited our hospital between April 2018 and March 2020 were included. All patients provided oral and written informed consent.
The average age of the patients was 63.4±14.0 years. The injury mechanisms of the patients were as follows: chainsaw injury (n=2), chronic skin ulcer (n=1), fasciotomy (n=1), snake bite (n=1), degloving injury (n=1) and delayed healing of surgical wounds (n=1) (Table 1).
Study design
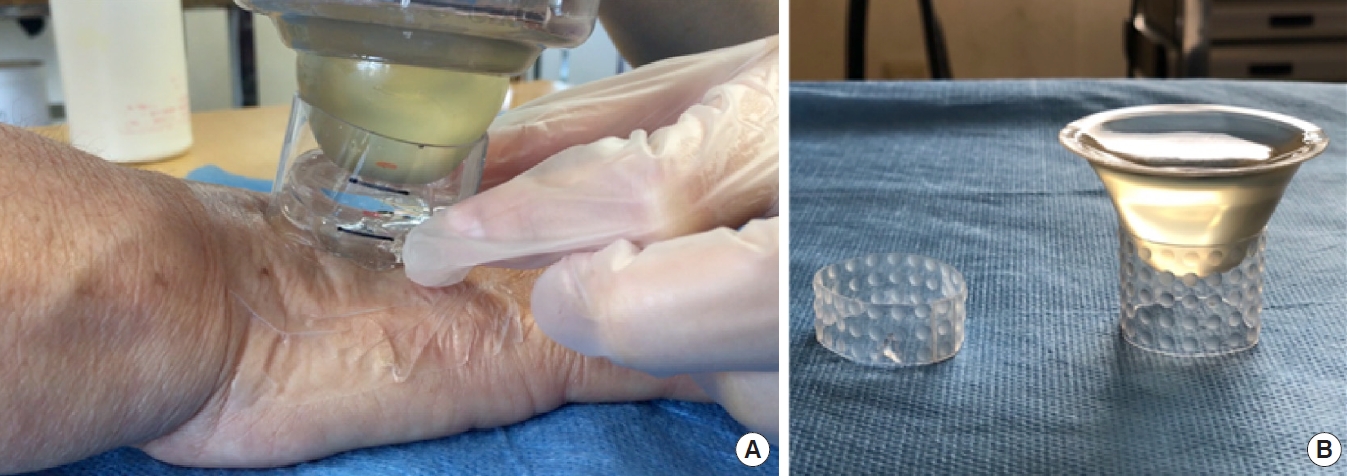
Before ESWT was performed, a K2 gel pod filled with jelly was used as a contact medium between the wound and ESWT apparatus. K2 gel pods were created using silicon sheets to bring the depth of focus of the ESWT energy closer to the surface. ESWT was performed using the Duolith SD1 shockwave system (Storz Medical AG) with a depth of focus using a stand-off distance of 15 mm, an energy flux density of 0.01–0.10 mJ/mm2, and 2,500 sessions per administration. ESWT was performed once a week for an average of 4.8 treatments. The energy flux density was initially 0.01 mJ/mm2. Energy flux density was gradually increased while confirming the site and degree of pain with the patient (biofeedback method). ESWT was performed in a spiral pattern from the edges of the wound toward the center.
The ESWT procedure is shown in Fig. 1. The wound was cleaned daily, and necrotic tissue was debrided if necessary; the wound was then covered with gauze. The wound was evaluated using the HFWAT, and scores were compared at three-time points: before ESWT, after three ESWT sessions, and at the final evaluation. Wilcoxon signed-rank test was used to compare the results. All statistical analyses were performed using a Python code run on SciPy 1.7.3 (Enthought, Inc.)
Hand and Foot Wound Assessment Tool
Wound assessment tools such as the Bates-Jensen Wound Assessment Tool [6,7] and DESIGN-R [8] have been previously reported. When using such wound evaluation tools for limb wounds, it is difficult to detect a difference in the score, as well as changes in scores according to wound size. The HFWAT was created to address these issues. With the HFWAT, the limbs are divided into areas, and the wounds are scored according to the ratio of the wound area to the total area, allowing for the assessment of small wounds. In addition, the definition of the score for each item is clarified. An advantage of the HFWAT is that examiner bias is less likely to occur.
The HFWAT comprises five wound assessment items: (1) area and size, (2) necrotic tissue and healthy granulation tissue, (3) depth, (4) exudate, and (5) inflammation and signs of infection (Table 2).
Area and size
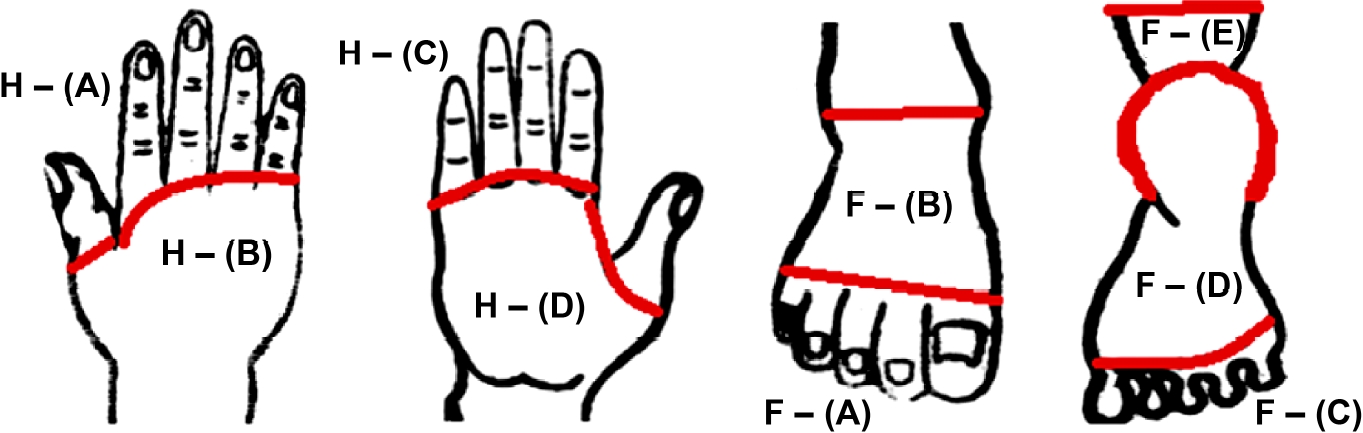
For the palm, the area was divided by the line connecting the palm-finger segment skin line on the palm side. The dorsal side was designated as H- (A) and H- (B), and the palm side was designated as H- (C) and H- (D). The dorsum of the foot was divided into areas according to the line connecting the metatarsophalangeal joints and the line connecting the lateral and medial malleolus, which were designated as F- (A) and F- (B). The plantar foot was divided into three areas, F- (C), F- (D), and F- (E), divided by the line connecting the metatarsophalangeal joints and the outer contour line of the plantar heel (Fig. 2). The score was then calculated according to the ratio of the wound area to the total area (Table 2). For wounds that extended over more than one area, the score for each area was given and the sum of the scores was used.
Necrotic tissue and healthy granulation tissue
The percentage of wound necrotic tissue and healthy granulation tissue was scored.
Depth
The depth of the wound was scored as follows: 0, without depth; 1, extending to the epidermis or dermis; 2, extending to the subcutaneous tissue, tissue necrosis, or muscle/tendon damage; and 3, extending to the bone.
Amount of exudate
The amount of exudate was scored by dividing it into small and large amounts according to the frequency of dressing changes. Wounds with small amounts of exudate were considered as “those that do not require daily dressing changes,” while those with large amounts of exudate were “those requiring dressing changes at least once a day.”
Inflammation/infection
Inflammation and infection of the wound were assessed and assigned 1 point in case of local inflammatory reactions (redness and swelling around the wound) and 2 points in case of obvious local signs of infection (pus and foul odor).
The total score of the above five items comprised the HFWAT score.
Results
Treatment details
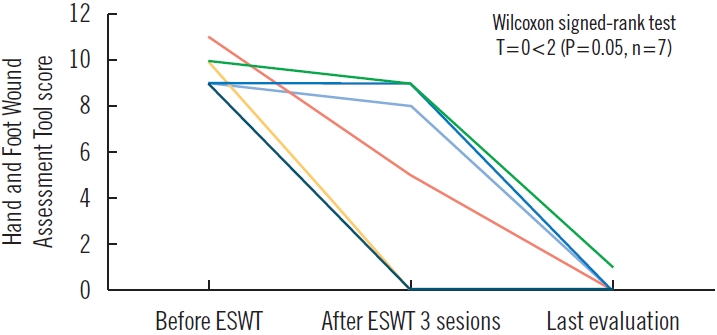
The average number of days from injury to the start of ESWT was 36.1±17.9, and the average treatment period was 27.0 days (Table 3). The average number of ESWT sessions was 4.8 (range, 3–8). An improvement in the median HFWAT score was noted: from 9.0 (interquartile range, 9–10) points before ESWT to 0.0 (interquartile range, 0–0) points at the final evaluation (P=0.05, n=7) (Fig. 3).
Case presentations
Case 1
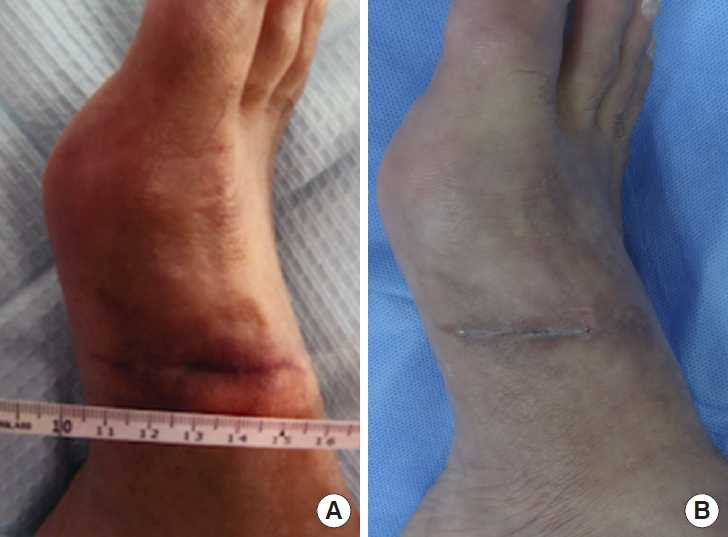
A 42-year-old man had a persistent skin ulcer on the back of his right foot for 6 months. The patient visited our hospital when the wound failed to heal even after using an ointment prescribed at another hospital. He complained of delayed wound healing and pain during walking. He had a history of right hemiplegia after cerebral infarction and collagen disease and was receiving steroids. ESWT was initiated 1 month after the first visit. The patient initially underwent three ESWT sessions. Proliferation of granules was observed following treatment. Complete wound healing was observed after six ESWT treatments, and pain improved. The HFWAT score improved from 9 points (size: 1, necrotic tissue: 4, depth: 1, exudate: 2, inflammation: 1) pre-ESWT to 0 points post-ESWT (Fig. 4).
Case 2
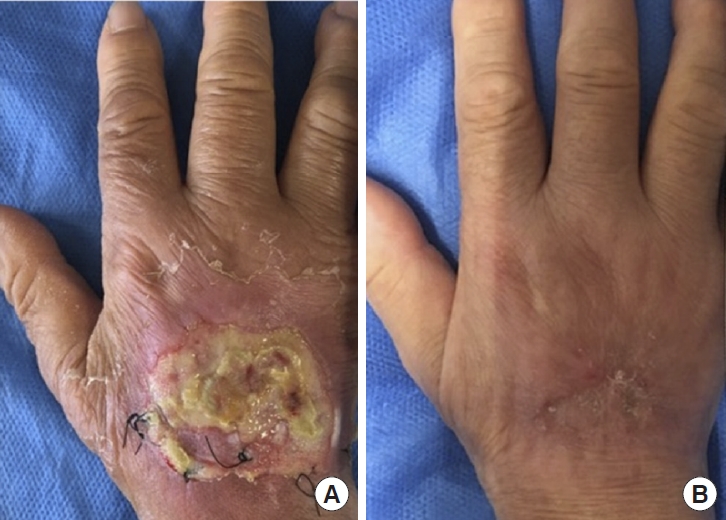
A 77-year-old woman was injured after her right hand was caught in a cardboard cutting machine. A degloving-like injury on the back of the right hand was observed, and the extensor tendon was exposed; however, there was no rupture or bone damage. The wound was washed and stitched with a nylon suture. An epidermal ulcer was observed 3 weeks after the injury. After five ESWT sessions, the wound healed completely. The HFWAT score improved from 11 points (size: 2, necrotic tissue: 4, depth: 2, exudate: 2, inflammation: 1) pre-ESWT to 0 points post-ESWT. Aggressive range-of-motion training was started 2 weeks after the injury. The patient had no restrictions at the final follow-up (Fig. 5).
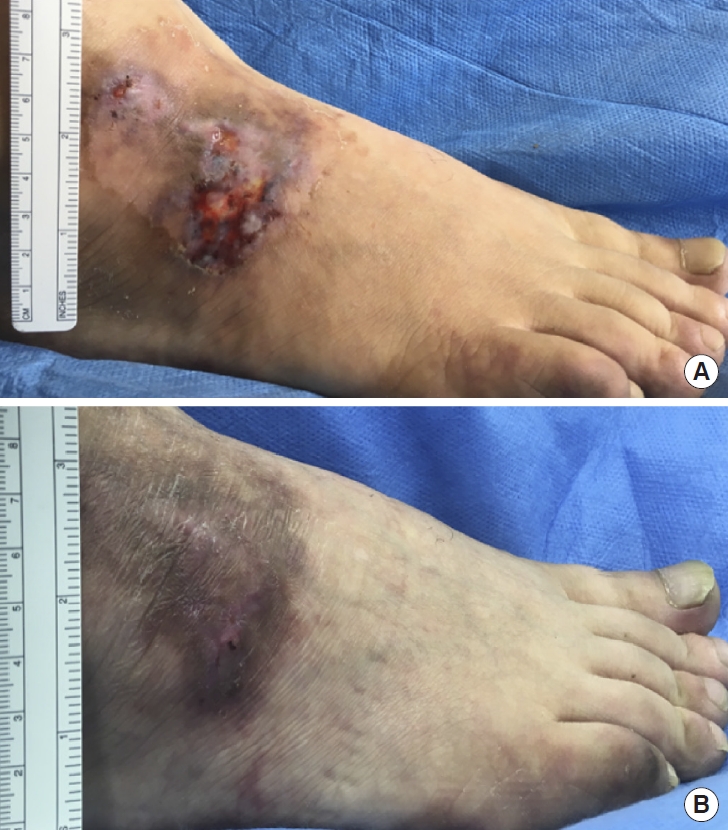
Case 3
A 63-year-old man injured the back of his right foot with a chainsaw. The extensor hallucis longus tendon was ruptured, and an open fracture of the metatarsal bone of the right toe was observed. The patient had a history of type 2 diabetes and high blood pressure. Three days after the injury, the wound to the extensor hallucis longus tendon was debrided and sutured. Percutaneous steel wire insertion was performed. The steel wire was removed 3 weeks after the injury, and the wound on the toe was secondarily sutured. No wound closure was observed 5 weeks after the secondary suture placement, and three ESWT sessions were performed. The wound healed 11 weeks after the injury. The HFWAT score improved from 9 points (size: 1, necrotic tissue: 3, depth: 2, exudate: 2, inflammation: 1) pre-ESWT to 0 points post-ESWT (Fig. 6).
Discussion
The mechanism of action of ESWT on skin wounds is thought to be angiogenic, induced by cavitation [9]. Cavitation is a phenomenon characterized by intracellular microbubble generation that disappears in the ischemic area of the vascular endothelium that has undergone low-power ESWT. This leads to a type of shear stress on the vascular endothelial cells, followed by increased expression of vascular endothelial growth factor, stromal cell-derived factors, and angiogenesis-promoting factors such as nitric oxide. These factors are thought to stimulate the secretion of vasodilators [9,10]. In addition, these effects are reportedly maximized at approximately 0.1 mJ/mm2, as reported by an in vitro study performed using cultured human vascular endothelial cells [9].
Low-output ESWT is reported to improve incision wound healing due to increased expression of transforming growth factor-β1 levels, including the release of cytokines, which stimulates collagen production to increase skin tensile strength [10]. On the other hand, when diabetic skin ulcers, pressure ulcers, and skin ulcers associated with vasculitis become chronic, the wound healing process may stagnate and be accompanied by bacterial infection. Use of ESWT is effective in combination with antibacterial agents. Shock waves are reported to be effective in destroying drug-resistant biofilms both in vitro and in vivo [11,12]; thus, it is suggested that ESWT may be useful for infected wounds.
ESWT has been reported to provide pain relief by destroying free nerve endings [13,14]. In case 1, the patient’s gait improved upon pain relief in the affected limb. Dysfunctions such as joint contracture and tendon adhesion are likely to remain when limb trauma occurs, even following minor trauma [5]. For degloving-like injuries such as that in case 2, ESWT could minimize the amount of wound-covering agent required, enabling wound treatment concomitantly with the range-of-motion training from the start of treatment.
In this study, we created and used our own tool, the HFWAT. As the limbs are divided into areas and scored by the ratio of the wound area to the total area, it was possible to analyze small wounds.
Falanga et al. [15] developed a wound assessment classification for the wound bed of lower extremity venous ulcers, termed the wound bed score, which is useful for prognosis evaluation in wound healing. However, the definition of the wound bed score may be ambiguous because the evaluation of items—such as the amount of exudate and dermatitis around the wound—comprises severe, moderate, and mild scores. By contrast, the HFWAT was developed to minimize bias across examiners by including defined criteria to score the exudate volume and inflammation, which conventional wound evaluation tools cannot determine. Additionally, the DESIGN-R [8] mentioned above is an assessment tool for pressure ulcers and is not easily adaptable to our cases.
Here, we used ESWT for refractory wounds and were able to achieve good results. As ESWT is a noninvasive treatment, it can be performed in patients with underlying diseases, such as diabetes or chronic kidney disease, as observed in some of our cases. However, for skin wounds, complications associated with ESWT occur owing to prolonged treatment time; these complications include pain and redness at the treated area, which can become severe.
According to the ISMST, careful consideration should be given to patients with contraindications wherein the pulmonary tissue, epiphyseal line, and peri-spine are located in the therapeutic area. We plan to continue providing ESWT and to improve this procedure. For ESWT, it is necessary to consider the frequency of treatment, timing of concomitant use of antibacterial agents in infected wounds, and cost of treatment.
This study has several limitations; first, it lacked a control group. The tendons, muscles, and other motor organs are directly under the skin of the extremities. If chronic wounds take time to heal, there is a risk of residual contractures. All patients in this study had been receiving other treatments for a long time and were at risk of contractures; it would, thus, be unethical to create a control group without ESWT. Second, the number of cases examined was small. Regarding the relative clinical utility of the HFWAT, no assessment tools specific to limb wounds exist, and given that the previously reported assessment tools for pressure and leg ulcers are not wholly applicable to such wounds, direct comparisons are difficult. This was, therefore, a preliminary study, and further studies must include a higher number of cases. Moreover, while treatment and evaluation were performed by two physicians and one physical therapist, we were unable to investigate inter-examiner bias. There is also a lack of basic evidence regarding the K2 gel pod used in ESWT; we aim to conduct further research to address these limitations in the future.
In this study, we described the effect of ESWT on intractable wounds and the results in our patients. We created the HFWAT to evaluate skin wounds on the extremities. Wound healing was observed in all cases, as indicated by significant improvements in the HFWAT scores obtained after ESWT treatment. In addition, dysfunction tends to persist if healing is prolonged in wounds on the extremities. No complications were observed in any patient.