 |
 |

|
| Aims and Scope |
| About the Journal |
| About the Society (KWMS) |
| Editorial Board |
| Open Access |
| Principles of Transparency and Best Practice |
| Editorial Office |
| Subscriptions |


|
| Aims and Scope |
| About the Journal |
| About the Society (KWMS) |
| Editorial Board |
| Open Access |
| Principles of Transparency and Best Practice |
| Editorial Office |
| Subscriptions |

AbstractBackgroundParticulate matter (PM) is known to increase the risk of diseases in major organs and PM may also slow the complex process of wound healing. We investigated the effect of PM on wound healing in vivo.
MethodsTwelve 10-week-old Sprague-Dawley male rats were punctured with three 8 mm-diameter holes each in the skin on their backs. The holes were divided into three groups according to treatment: PM 50 µg/mL (group A), PM 100 µg/mL (group B) and saline (control). For histologic analysis, three rats were sacrificed on each of the 1st, 3rd, 7th, and 28th days, and a total of 36 samples were collected. Inflammatory cell infiltration and neovascularization were evaluated for comparison among groups.
ResultsInflammation increased in groups A and B on day 1, but no significance was observed. On the 28th day, increased inflammation was observed in groups A and B compared to the control group, with significant difference. Angiogenesis increased in groups A and B compared to the control group on day 1, but no significance was observed. On the 3rd day, there was a decrease in group B, with statistical significance. On day 28, it was observed that angiogenesis significantly increased in group B compared to the control group.
IntroductionParticulate matter (PM) in the air is a complex mixture of particles and chemicals, and the particle size, concentration, and chemical content of PM vary widely. It is composed of a wide range of chemical elements including nitrates, sulfates, hydrocarbons, organic compounds, biological chemicals, and metals [1]. In general, PMs are classified according to size, and to date, particles with a diameter of less than 10 µm have been identified as having the greatest impact on human health [2,3].
There has been continuous epidemiological research showing that airborne PM produces a variety of negative health outcomes including respiratory and cardiovascular disorders [4]. As indicated by the World Health Organization, PM exposure accounts for approximately 8×105 unexpected deaths every year by causing cardiovascular and respiratory illnesses, and PM exposure has a higher impact than exposure to any other pollutant [5]. Studies have reported that the causes of these diseases are genotoxic, mutagenic, and carcinogenic effects of PM [6].
Wound healing is a normal biological process of skin tissues in the body [7]. For wound healing, a well-coordinated, integrated, complex process is required, including hemostasis, inflammation, proliferation, and tissue remodeling [8]. There are many factors that may affect wound healing, interfering with one or more phases of this process, thus causing infection or chronic wounds. PM includes a variety of chemical and biological substances that might induce inflammation-related damage by upregulating the expression of numerous inflammatory pathways, cytokines, and genes [9].
While pollutants such as tobacco smoke and air pollution have been intensively studied for their harmful effects on human skin, the effects of PM on wound healing have not been studied. Therefore, we hypothesized that PM would inhibit wound healing and investigated the effect of PM in in vivo models.
MethodsPreparation of PM4.0Fine PM NIST 2786 (PM4.0) was purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). A PM4 stock solution diluted with 0.5 mL normal saline was prepared, and was sonicated to avoid agglomeration of the suspended PM4 particles.
Animal preparationTwelve 10-week-old male Sprague-Dawley rats weighing 330 g were used in this study. After a 1-week acclimatization period, the rats were anesthetized with sevoflurane for inhalation of anesthesia. The fur was shaved using an electric shaver and disinfected with 10% povidone-iodine and 70% alcohol solutions. The animal experiments were approved by the Institutional Animal Care and Use Committee of Wonkwang University (IACUC No. WKU21-48).
Wound generationA biopsy punch (Kai Medical, Chiyoda, Japan) was used to create three 8 mm physical holes in the skin on the back of the rats. Normal saline (control), PM 50 µg/mL (group A), and PM 100 µg/mL (group B) solutions were applied to each of the three holes of the rats. The wounds were then covered with a transparent film dressing (Tegaderm; 3M, Saint Paul, MN, USA) (Fig. 1). The dressing was confirmed every day and was replaced only if damaged.
Histology and histomorphometric analysesTo observe histological changes, three rats were euthanized on each of days 1, 3, 7, and 28. Tissue samples were collected for each hole, and a total of 36 samples were subjected to analysis. The tissues were collected to include the normal tissue surrounding the defects. They were fixed with 10% formalin solution, embedded in paraffin, cut into slices, and stained with hematoxylin and eosin (H&E) and Masson’s trichrome stains. The stained slides were scanned on a digital slide scanning device, and the images were visualized using CaseViewer software version 2.4 (3DHISTECH, Budapest, Hungary). A descriptive analytical study of the inflammatory infiltrate and neovascularization was performed and a comparative analysis among groups was carried out. The penetration of polymorphonuclear and mononuclear cells was presented as a morphological score of inflammation, and the new vascular formation of the tissues was measured for scoring angiogenesis. The data were obtained using a semi-quantitative scoring system described previously [10,11]. Inflammation was scored as follows: 0, absence of inflammation; 1, presence of few inflammatory cells; 2, many inflammatory cells; and 3, exaggerated inflammatory cellularity. Angiogenesis was scored similarly: 0, normal vascularization; 1, discrete vascular formation; 2, moderate vascular formation; and 3, high vascular formation.
The histological sections were examined by two examiners, who were blinded to the group information while performing all the histological analyses in this study, and their average score value was used for the comparative tissue analysis.
Statistical analysisStatistical data and graphs were generated via performing analyses of variance using StatView software (SAS Institute Inc., Cary, NC, USA). Values were expressed as the mean±standard deviation. The significance of the differences between groups was determined by using Fisher’s least significant difference post-hoc analyses.
ResultsAt 4 weeks after wound generation, all three groups were in a completely healed state, and showed no complications such as infection.
Inflammation increased in groups A (3.00±0.00) and B (2.83±0.41) compared to that in the control group (2.50±0.93), and angiogenesis increased in group A (2.00±1.16) compared to that in the control group (1.00±0.00) and group B (1.00±0.00), but the difference was not significant on day 1. Three days after injecting PM, angiogenesis decreased in group B (2.14±1.07) compared to that in the control group (2.88±0.35) and group A (2.88±0.35), and the difference was significant (P=0.029). Seven days after injecting PM, angiogenesis decreased in group A (2.50±0.76) compared to that in the control group (3.00±0.00), but the difference was not significant (P=0.105). Lastly, after 28 days of injecting PM, inflammation increased in groups A (2.00 ±0.00) and B (1.50±0.55) compared to that in the control group (1.00± 0.00), and the difference was significant (P<0.001, P=0.027). Angiogenesis also tended to increase in groups A (0.50±0.58) and B (1.83±0.98) compared to that in the control group (0.50±0.55), and the difference was significant only in group B (P=0.004) (Tables 1 and 2).
H&E staining (Fig. 2) showed that inflammation tended to increase while angiogenesis tended to decelerate.
DiscussionAir pollution, which is widespread globally, has an increasingly harmful effect on human health [12]. PM is an important part of air pollution and has been shown to increase the risk of health-threatening diseases [13]. PM can be classified according to its aerodynamic diameter as PM0.1 (ultrafine particle, <0.1 µm), PM2.5 (fine particle, <2.5 µm), and PM10 (<10 µm) [2,3,14]. PM is composed of pollutants such as metals, carbon, sulfates, nitrates, and other inorganic ions, which come from industrial and domestic coal burning as well as emissions from diesel and petrol engines [9,15,16]. Exposure to PM, even for a few hours, can increase the risk of mortality due to cardiovascular disease or other non-lethal events. Long-term effects of PM have been studied, and the association between PM exposure and the occurrence of asthma, chronic bronchitis, and ischemic heart disease has been reported. In addition, long-term exposure to PM is associated with reduced life expectancy [17]. While the influence of airborne PM on the respiratory and cardiovascular systems in humans has received most of the attention, many other systems are also affected by repeated exposure to PM [18-20].
There are four main mechanisms through which PM adversely affects human health: oxidative stress, genotoxicity, cell death, and inflammation [6]. Among those mechanisms, inflammation is a common cause of various diseases related to PM exposure [9]. In the respiratory system, it is known that PM causes important inflammatory responses initiated by alveolar macrophages and airway epithelial cells. These cells, after phagocytosing PM, produce pro-inflammatory mediators that contribute to the lung immune response, and lead to oxidative stress and systemic inflammation [9,21]. Although the specific mechanism by which PM exposure causes cardiovascular disease has not yet been fully understood, the inflammatory response to PM has a negative impact on the cardiovascular system by producing epigenetic alterations contributing to microvascular dysfunction, endothelial damage, and poor coagulation, as well as affecting the autonomic response [9,22]. Lastly, in the central nervous system, PM induces neuroinflammation by translocating to the brain parenchyma through olfactory nerve or systemic circulation, resulting in destruction of the blood brain barrier or by indirect pathways from pro-inflammatory cytokines of nasal epithelium or systemic circulation [23].
Wound healing is a normal but complex process, involving the phases of hemostasis, inflammation, proliferation, and remodeling. Each phase must proceed reliably and with consistency, and if abnormal progression occurs at any one phase, wound healing may be prolonged or the wound may become chronic. A number of different types of cells are involved in these phases [24]. Because wound healing is complex and sequential, precise evaluation and treatment for each phase is a major target in therapeutic approaches. Clinical features and biochemical and histological parameters are used to measure wound healing; histological parameters can include angiogenesis, inflammation, wound contraction, epithelialization, and differentiation [25].
In consideration of the aforementioned, we conducted an experiment assuming that PM could affect the wound healing process, especially in the inflammation phase. We performed this study using an in vivo experimental model. After 28 days of PM injection, inflammation increased in the PM group compared to that in the control saline group. Moreover, angiogenesis was delayed in the PM group.
This increased inflammation is believed to be caused by prolonged elevation of pro-inflammatory cytokines such as interleukin-1 and tumor necrosis factor-α. In vitro and in vivo studies have shown that this increase in pro-inflammatory cytokines can be caused by PM [9,21].
This study had several limitations as a preliminary study. First, because the physiological structures of rats are different from those of humans, it is questionable whether the study results could be generalized to humans. Two- and three-dimensional in vitro human skin models could be excellent alternatives to verify this. Second, the study results were based only on histological findings, and further investigations such as immunohistochemical studies and enzyme-linked immunosorbent assays are required to determine more accurate associations between PM exposure and wound healing. Third, we used only PM4 in this study; therefore, it is necessary to investigate the relevance according to the size of the PM. Finally, despite the fact that some data values were not statistically significant, differences between each group could be observed. Missing values or overestimation of the number of microvascular structures on H&E staining because of preexisting vessels can be considered as the cause of the difference in statistical significance. If in vitro and more diverse studies are conducted and better results are obtained on the effect of PM on wound healing, then it will greatly contribute to providing solutions to protect against PM exposure.
In conclusion, while previous studies have reported various harmful effects of PM on human health, this study is meaningful in that it is the first study on the effect of PM on wound healing. The present study revealed that PM affects wound healing by increasing inflammation and delaying angiogenesis. The results can support further investigation on the effect of PM on wound healing.
Conflict of InterestThis work was supported by Wonkwang University in 2021. Young Cheon Na is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported. Fig. 1.Wound creation on back of rat. (A) Wounds were created using a biopsy punch to make three 8 mm-diameter holes. (B) The wounds were covered with a transparent film dressing after introducing saline (control), PM 50 µg/mL (group A), and PM 100 µg/mL (group B) in each punch hole. 
Fig. 2.Histologic findings. The particulate matter-contaminated groups A and B both displayed increased inflammatory scores and delayed angiogenesis compared to the control group (H&E, ×20). 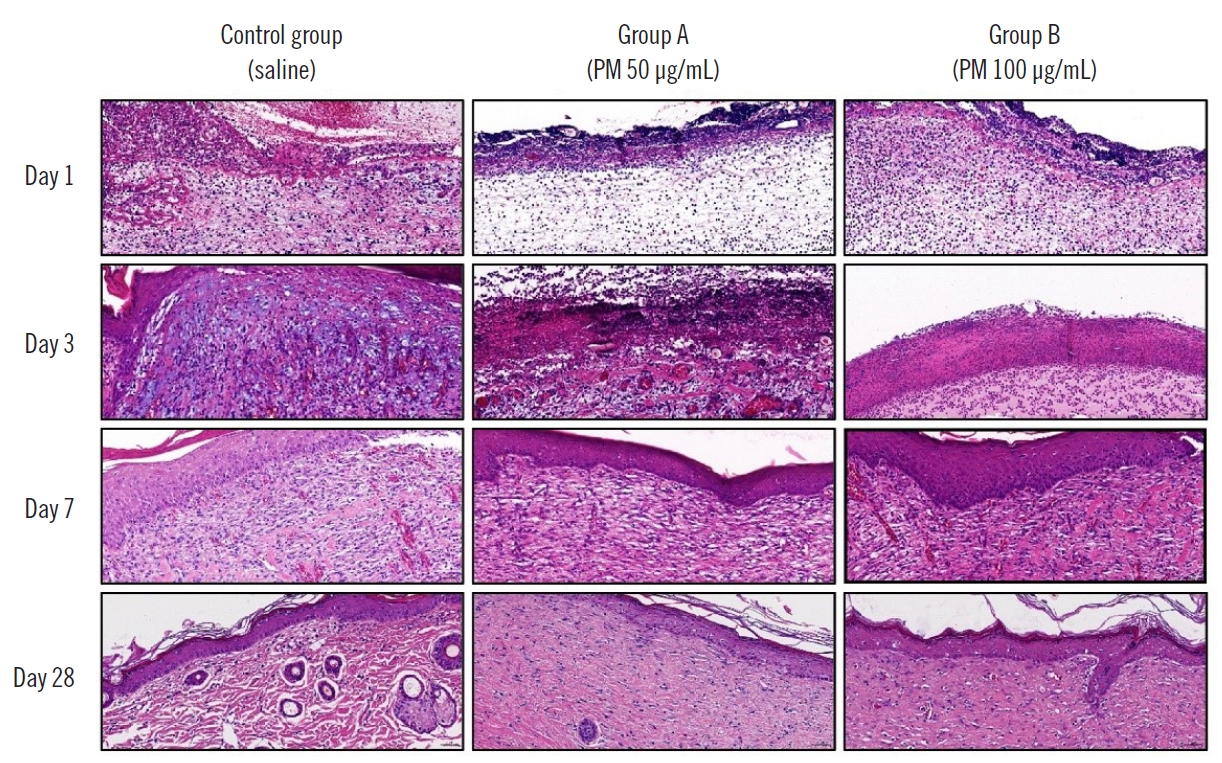
Table 1.Comparison of inflammation progression using the semi-quantitative scoring system
Table 2.Comparison of angiogenesis progression using the semi-quantitative scoring system
References2. United States Environmental Protection Agency. Integrated review plan for the National Ambient Air Quality Standards for Particulate Matter. Research Triangle Park: United States Environmental Protection Agency; 2008.
4. Laumbach RJ, Kipen HM. Respiratory health effects of air pollution: update on biomass smoke and traffic pollution. J Allergy Clin Immunol 2012;129:3-11.
5. Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. J Med Toxicol 2012;8:166-75.
6. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224-60.
7. Herdrich BJ, Lind RC, Liechty KW. Multipotent adult progenitor cells: their role in wound healing and the treatment of dermal wounds. Cytotherapy 2008;10:543-50.
8. Shin VY, Liu ES, Koo MW, et al. Cigarette smoke extracts delay wound healing in the stomach: involvement of polyamine synthesis. Exp Biol Med (Maywood) 2002;227:114-24.
9. Arias-Perez RD, Taborda NA, Gomez DM, et al. Inflammatory effects of particulate matter air pollution. Environ Sci Pollut Res Int 2020;27:42390-404.
10. de Moura Estevao LR, Cassini-Vieira P, Leite A, et al. Morphological evaluation of wound healing events in the excisional wound healing model in rats. Bio Protoc 2019;9:e3285.
11. Alpdundar Bulut E, Bayyurt Kocabas B, Yazar V, et al. Human gut commensal membrane vesicles modulate inflammation by generating M2-like macrophages and myeloid-derived suppressor cells. J Immunol 2020;205:2707-18.
12. World Health Organization. Health effects of particulate matter: policy implications for countries in eastern Europe, Caucasus and central Asia. Copenhagen: World Health Organization; 2013.
13. Beelen R, Hoek G, van den Brandt PA, et al. Long-term exposure to traffic-related air pollution and lung cancer risk. Epidemiology 2008;19:702-10.
14. Ayrault S, Senhou A, Moskura M, et al. Atmospheric trace element concentrations in total suspended particles near Paris, France. Atmos Environ 2010;44:3700-7.
15. Kim Oanh NT, Batz Reutergardh L, Dung NT. Emission of polycyclic aromatic hydrocarbons and particulate matter from domestic combustion of selected fuels. Environ Sci Technol 1999;33:2703-9.
16. Jung S, Lim J, Kwon S, et al. Characterization of particulate matter from diesel passenger cars tested on chassis dynamometers. J Environ Sci (China) 2017;54:21-32.
17. Brook RD, Rajagopalan S, Pope CA 3rd, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 2010;121:2331-78.
18. Newby DE, Mannucci PM, Tell GS, et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J 2015;36:83-93b.
19. Holgate ST. ‘Every breath we take: the lifelong impact of air pollution’: a call for action. Clin Med (Lond) 2017;17:8-12.
20. Schraufnagel DE, Balmes JR, Cowl CT, et al. Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2. Air pollution and organ systems. Chest 2019;155:417-26.
21. Yang B, Chen D, Zhao H, et al. The effects for PM2.5 exposure on non-small-cell lung cancer induced motility and proliferation. Springerplus 2016;5:2059.
22. Pope CA 3rd, Bhatnagar A, McCracken JP, et al. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res 2016;119:1204-14.
|
|