Introduction
Excessive intraoperative blood loss can worsen major perioperative complications [1]. Therefore, surgeons use several methods to stop bleeding in the surgical field. Topical hemostatic agents can be used when conventional hemostatic methods, such as compression, ligation, and electrocauterization are impractical and unyielding [2,3]. For example, the conventional methods may not be adequate to maintain hemostasis in friable vessels or tissues containing diffuse capillaries and bony surfaces [1,4]. In such cases, topical hemostatic agents can be highly effective. However, topical hemostatic agents can interfere with wound healing by causing foreign body reactions, inflammation, and infection [3]. Therefore, surgeons should consider the effect of a hemostatic agent on wound healing before selection.
Gentamicin collagen sponge (Genta-Coll), manufactured by Resorba (Nürnberg, Germany), is a topical hemostatic material that also provides antibiotic protection against infections. However, it can also interfere with the wound healing process, as explained above. Therefore, we performed two examinations as presented below to evaluate the effect of Genta-Coll on the wound healing process as a topical hemostatic agent to guide surgeons in selecting topical hemostatic agents.
In the first examination, we aimed to investigate the effect of Genta-Coll on the wound healing process. Since human and rat skin structures are very similar from the outer layer to the epithelial appendages [5], we tried creating a skin defect on the rat abdomen where Genta-Coll could be placed, with the goal of assessing its effect. However, it was difficult to keep these materials on the wound site until the examination was finished. Therefore, instead of creating a skin defect, we created a muscle defect on the rectus abdominis muscle in rats, applied a Genta-Coll patch, and covered it using skin to assess its effect. Second, we also performed a zone of inhibition test to analyze the sensitivity of pathogenic bacteria to antimicrobial compounds and the ability to prevent surgical site infections (SSIs) by placing Genta-Coll in Mueller–Hinton agar plate seeded with nosocomial bacteria that most commonly cause SSIs.
Methods
Study 1: effect of Genta-Coll on wound healing
Experimental animals
The experimental protocol, including the use of animals in this study, was approved by the Institutional Animal Care and Use Committee of Wonkwang University (IACUC No. WKU 21-64). In total, 15 white male Sprague Dawley rats, 9 weeks old, weighing between 320 to 360 g, grown under the same living conditions were used in this experiment. The study was started after an acclimatization period of 1 week in the laboratory. The rats were provided a food pellet diet and water throughout the experiment. We used Genta-Coll with dimensions of 5×5×0.5 cm, containing 70 mg of collagen and 50 mg of gentamicin sulfate.
Wound induction
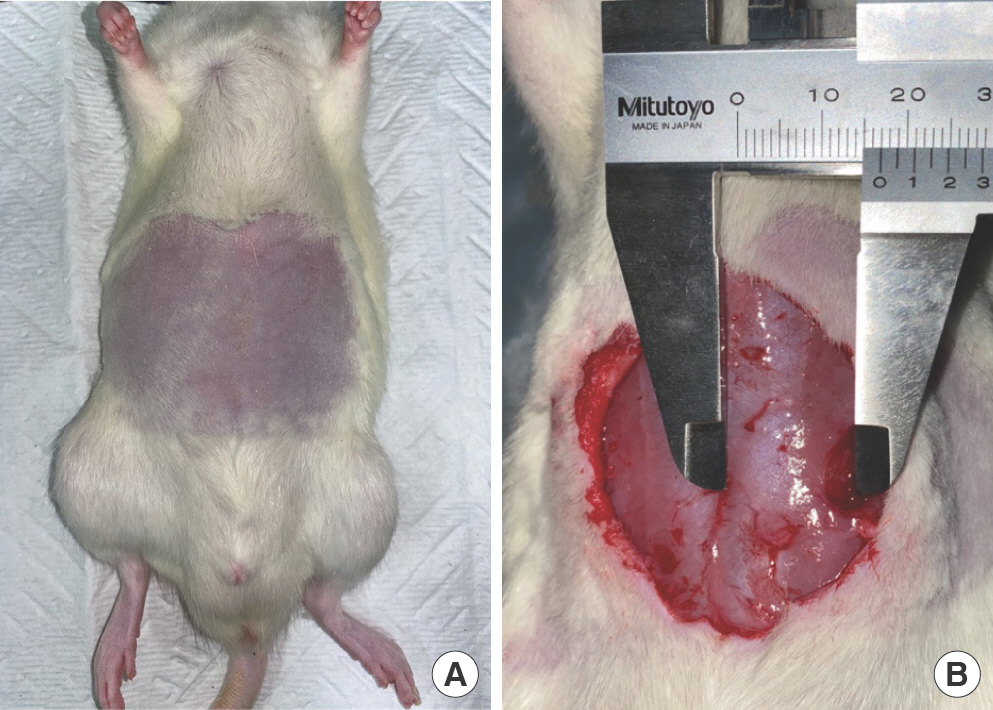
Isoflurane and sevoflurane (Baxter Healthcare Corp., Deerfield, IL, USA) were used for inhalation anesthesia. Abdominal hair was removed using an electric shaver, and the abdomen was disinfected using 10% povidone-iodine and 70% alcohol solution (Fig. 1A). The midline of the abdominal wall was incised, and the subcutaneous tissue was separated using a No. 15 scalpel and scissors. Subsequently, a disposable 8-mm-di-ameter biopsy punch (Cat. No: MT3337, Miltex Inc., Boerne, TX, USA) was used to create an 8×8 mm round shape defect in both rectus abdominis muscles, 15 mm away from the midline (Fig. 1B).
Surgical procedure and postoperative care
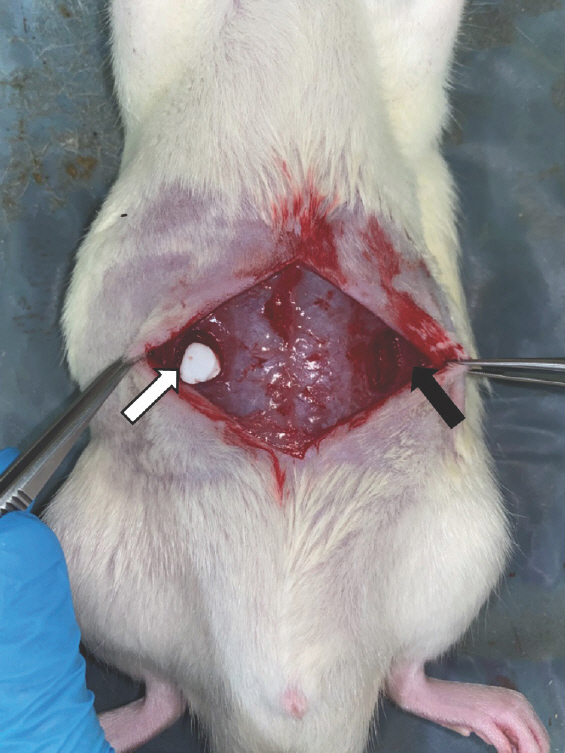
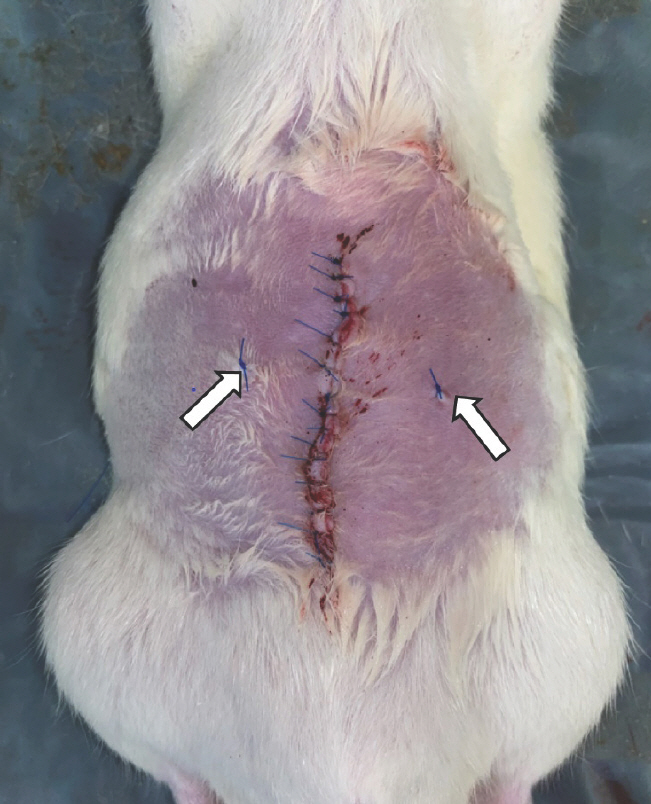
The rats were randomly divided into three groups of five animals each, to undergo biopsies on either day 3, day 7, or day 27. The rectus defects of each rat were divided into two groups for biopsy: group A and control group. The muscle defects in group A were treated using Genta-Coll according to the manufacturer's instructions, including dry implantation and loose insertion (Fig. 2). The control group did not receive any special care for the muscle defect. After applying Genta-Coll on one site of the muscle defect and leaving the other defect without care, the biopsy location was marked using a 4-0 Prolene suture, and the incision site was closed with a 5-0 nylon suture (Fig. 3). The suture site was disinfected with 10% povidone-io-dine and 70% alcohol. After completing the operation, administration of food pellet diet and water was continued for 27 days.
Wound biopsy
The tissues of the previously induced muscle defect areas and surrounding normal tissue were collected from both rectus abdominis muscles (group A and control group) by sacrificing the rats on the 3rd, 7th, and 27th postoperative days. The tissue samples from each rat were fixed in 10% formalin solution and stained with hematoxylin and eosin as well as Masson's trichrome staining according to the standard protocol.
Histologic evaluation
An optical microscope was used for histological analysis. Group A (Genta-Coll) was compared against the control group for various parameters listed in Table 1. The pathologist quantified each parameter by scoring using a semi-quantitative scoring system.
Table 1.
Seven parameters for histological analysis
Statistical analysis
We screened for the parameters in Table 1 to analyze the differences in wound healing in the two groups. The results are expressed as means±standard deviations. The Mann-Whitney test was conducted in each group. Statistical data were generated by using SPSS version 27.0 software (IBM Corp., Armonk, NY, USA). The level of significance was set at P<0.05.
Study 2: effectiveness of Genta-Coll against nosocomial bacteria
Materials and methods
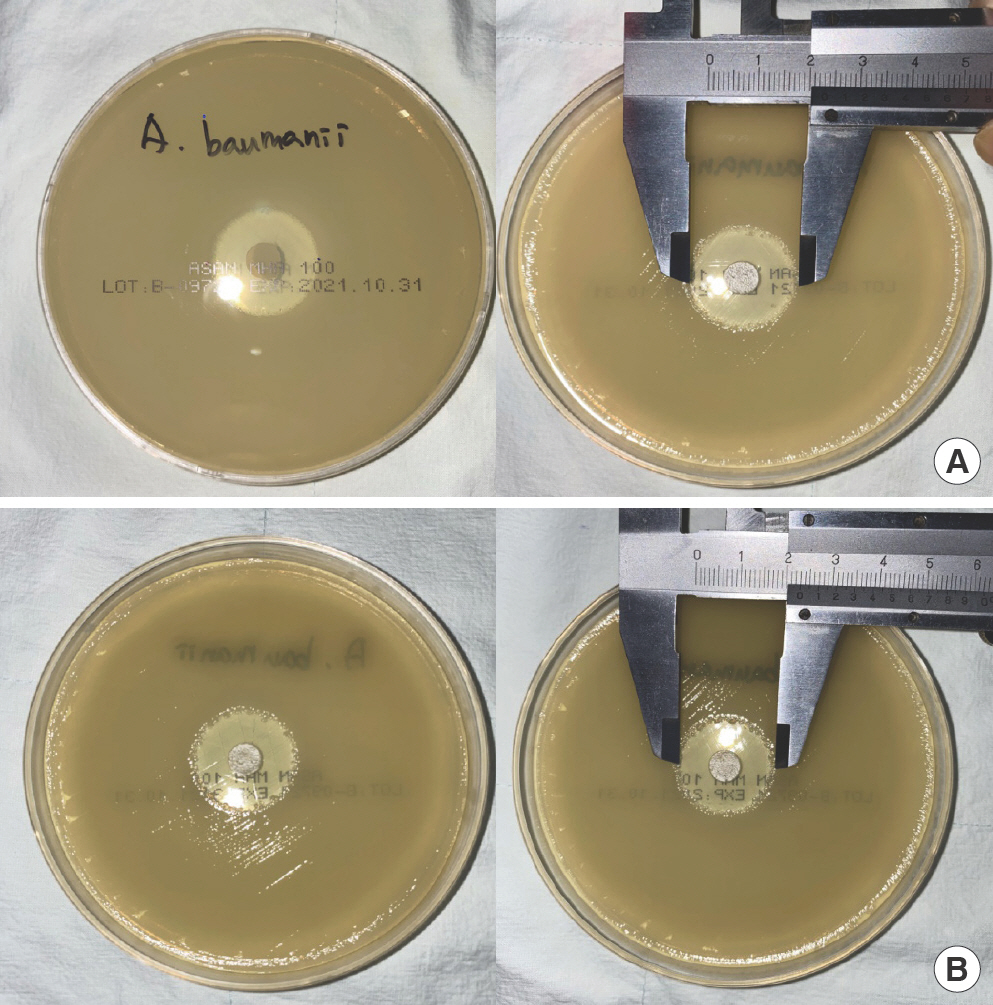
The following microorganisms, commonly identified in SSIs, were cultured: methicillin-sensitive Staphylococcus aureus (MSSA), methicillin-resistant S. aureus (MRSA), Acinetobacter baumannii, Escherichia coli, Enterococcus faecalis, Pseudomonas aeruginosa, and Candida albicans [6,7]. After inoculating each microorganism in each Mueller–Hinton agar plate, the microorganisms were incubated at 37°C for 24 hours. After 24 hours, a single colony from each agar plate was transferred using a sterile cotton swab to a 0.45% normal saline solution to check the turbidity against a 0.5 McFarland standard, except for C. albicans, as required to match with 2.0 McFarland standard, in line with standard experimental protocol (Fig. 4). After achieving the required turbidity, new Mueller–Hinton agar plates were inoculated with each microorganism, and 8-mm diameter Genta-Coll disks, identical to those used in the rat experiment in terms of material, size, and shape, were placed at the center of the agar plates using sterilized forceps (Fig. 5). The plates were incubated for 48 hours. Five plates were cultured for each bacteria using the aforementioned method. The diameter of the zone of inhibition was measured twice, after 24 (Fig. 6A) and 48 hours (Fig. 6B), using a ruler to the nearest millimeter. The mean diameter of thezone of inhibition was calculated, and the values were compared to analyze the effectiveness of Genta-Coll against nosocomial bacteria.
Results
Study 1: effect of Genta-Coll on wound healing
Histologic evaluation–postoperative day 3
Inflammatory cell infiltration, granulation tissue, fibroblast proliferation, neovascularization, extracellular matrix (ECM) formation, and striated muscle repair were observed in both groups. Inflammatory cell infiltration (2.70±0.45), granulation tissue (2.00±1.00), and neovascularization (2.80±0.45) were significantly higher in group A than in the control group (2.50±0.50, 1.60±0.55, and 2.60±0.55, respectively); however, the differences were not significant (P=0.496, P=0.502, P= 0.513, respectively). Striated muscle repair was markedly higher in the control group (1.00±1.00) than in group A (0.60±0.55); however, the difference was not significant (P=0.502). Fibroblast proliferation and ECM formation were similar in both groups (Table 2, Fig. 7).
Table 2.
Parameters of wound healing (day 3)
| Parameter | Control group | Group A a) | P-value |
|---|---|---|---|
| Foreign body giant cell | 0.00±0.00 | 0.00±0.00 | - |
| Inflammatory cell infiltration | 2.50±0.50 | 2.70±0.45 | 0.496 |
| Granulation tissue | 1.60±0.55 | 2.00±1.00 | 0.502 |
| Fibroblast proliferation | 2.00±0.00 | 2.00±0.00 | - |
| Neovascularization | 2.60±0.55 | 2.80±0.45 | 0.513 |
| Extracellular matrix formation | 1.20±0.45 | 1.20±0.45 | - |
| Striated muscle repair | 1.00±1.00 | 0.60±0.55 | 0.502 |
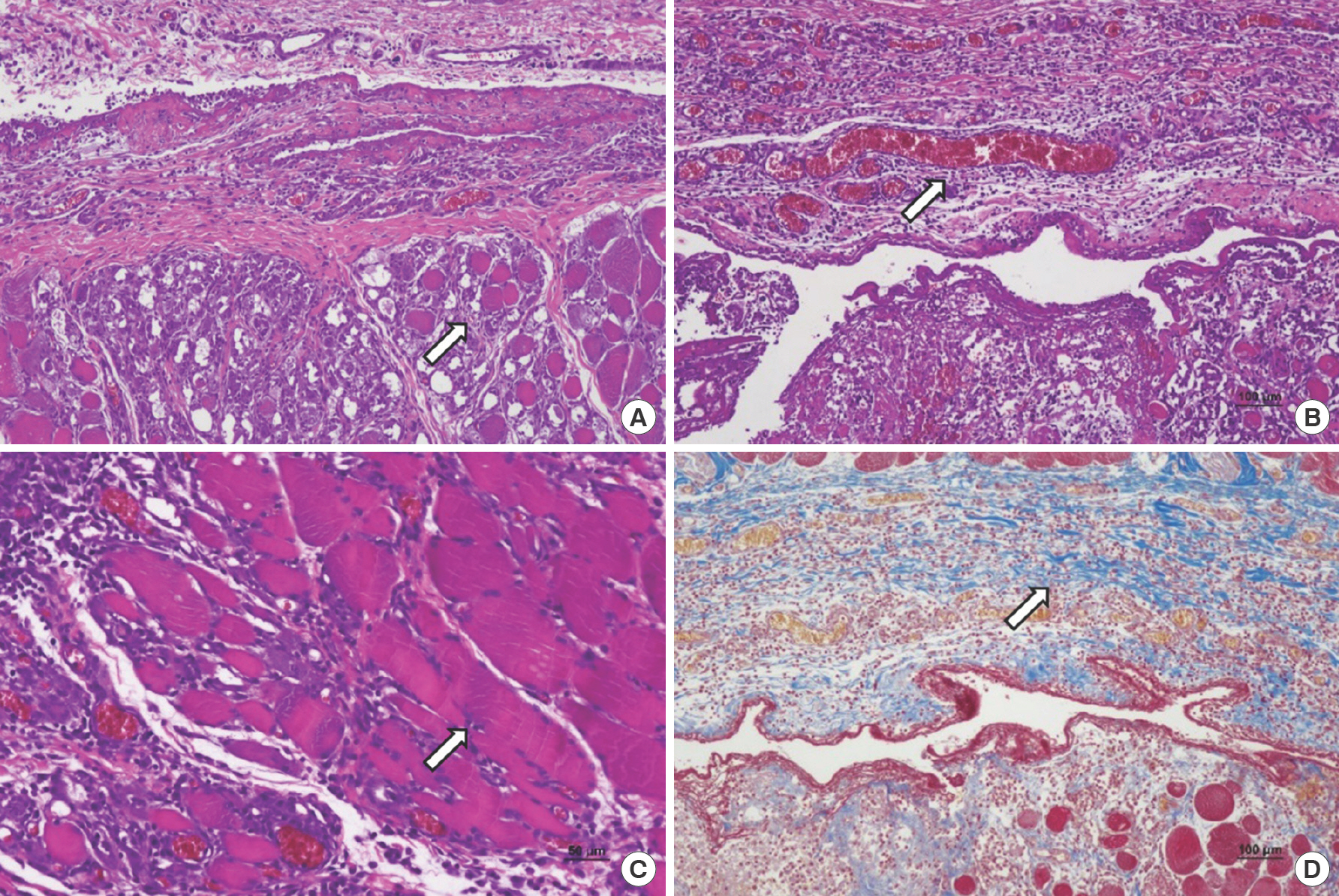
Fig. 7.
Three days after wound induction. (A) Control group, inflammatory cell infiltration, granulation tissue, fibroblast proliferation, neovascularization, extracellular matrix formation, and striated muscle repair (arrow) were observed (H&E, ×100). (B) In group A (treated with gentamicin collagen sponge), inflammatory cell infiltration, granulation tissue, and neovascularization (arrow) were higher than the control group (H&E, ×100). (C) Control group, striated muscle repair (depicted by arrow), showed a higher score compared to group A (H&E, ×100). (D) Extracellular matrix formations (arrow) were similar in both groups in Masson's trichrome stain (×100).
Histologic evaluation–postoperative day 7
Foreign body giant cells (2.20±0.84), granulation tissue (2.00±1.00), fibroblast proliferation (2.80±0.45), ECM formation (2.00±0.71), and striated muscle repair (1.20±1.64) increased in the control group compared to that observed on day 3. These parameters had also aggravated in group A, and a significant increase in muscle regeneration was observed in group A (2.75±0.43) than in the control group (1.20±1.42) (P=0.041). Foreign body giant cell scores were higher in the control group (2.20±0.84) than in group A (1.80±0.45) on day 7; however, the difference was not significant (P=0.343). ECM formation was similar in both groups (Table 3, Fig. 8).
Table 3.
Parameters of wound healing (day 7)
| Parameter | Control group | Group A a) | P-value |
|---|---|---|---|
| Foreign body giant cell | 2.20±0.84 | 1.80±0.45 | 0.343 |
| Inflammatory cell infiltration | 1.50±0.50 | 1.90±0.55 | 0.214 |
| Granulation tissue | 2.00±1.00 | 2.40±0.55 | 0.502 |
| Fibroblast proliferation | 2.80±0.45 | 2.60±0.55 | 0.513 |
| Neovascularization | 2.00±1.00 | 2.40±0.55 | 0.502 |
| Extracellular matrix formation | 2.00±0.71 | 2.00±0.71 | - |
| Striated muscle repair | 1.20±1.42 | 2.75±0.43 | 0.041 |
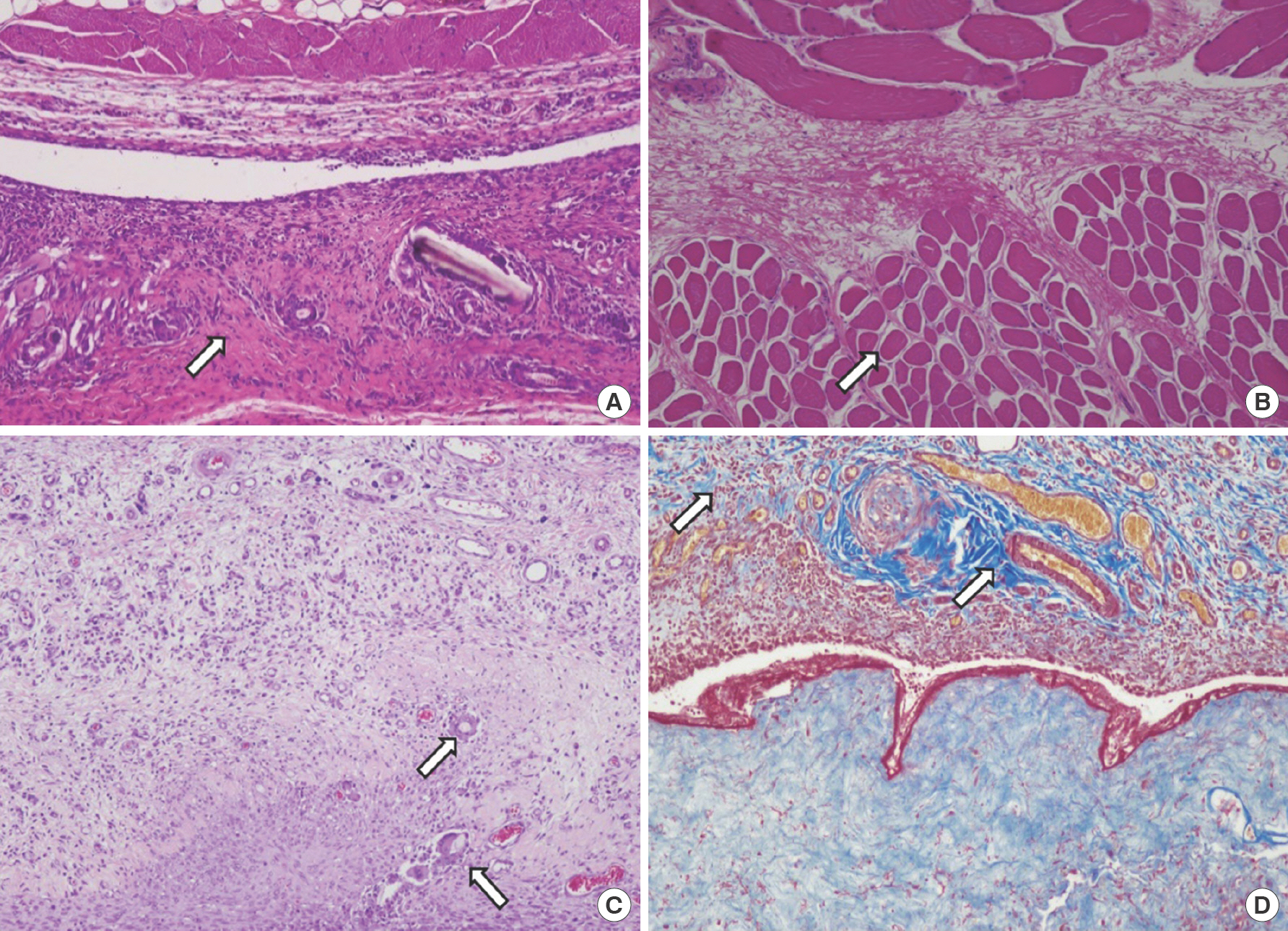
Fig. 8.
Seven days after wound induction. (A) Control group, foreign body giant cell, granulation tissue, fibroblast proliferation, extracellular matrix formation, and striated muscle repair increased compared to day 3. Moreover, we could find sufficient granulation tissue formation (arrow) (H&E, ×100). These parameters also increased in group A (treated with gentamicin collagen sponge). (B) In group A, muscle regeneration increased significantly (arrow) compared to the control group (H&E, ×100). (C) Control group, foreign body giant cells (arrow) increased compared to group A (H&E, ×100). (D) Extracellular matrix formation (arrow) was similar in both groups as observed after Masson's trichrome staining (×100).
Histologic evaluation–postoperative day 27
In both groups, inflammatory cell infiltration showed a comparable reduction, indicating that wound healing had been completed. Most parameters were on similar levels in both groups, except for foreign body giant cells and striated muscle repair. Although foreign body giant cell counts were higher in group A (2.40±0.89) than in the control group (1.80±0.84), the difference was not significant (P=0.268). However, the number of inflammatory cells was nearly the same in both groups. The striated muscle repair continued until 27 days in group A (1.00±1.41). ECM formation was similar in both groups (Table 4, Fig. 9).
Table 4.
Parameters of wound healing (day 27)
| Parameter | Control group | Group A a) | P-value |
|---|---|---|---|
| Foreign body giant cell | 1.80±0.84 | 2.40±0.89 | 0.268 |
| Inflammatory cell infiltration | 0.10±0.22 | 0.20±0.27 | 0.513 |
| Granulation tissue | 1.20±0.45 | 1.20±0.45 | - |
| Fibroblast proliferation | 1.60±0.55 | 1.40±0.55 | 0.549 |
| Neovascularization | 1.80±0.45 | 2.00±0.00 | 0.317 |
| Extracellular matrix formation | 3.00±0.00 | 3.00±0.00 | - |
| Striated muscle repair | 0.60±0.89 | 1.00±1.41 | 0.723 |
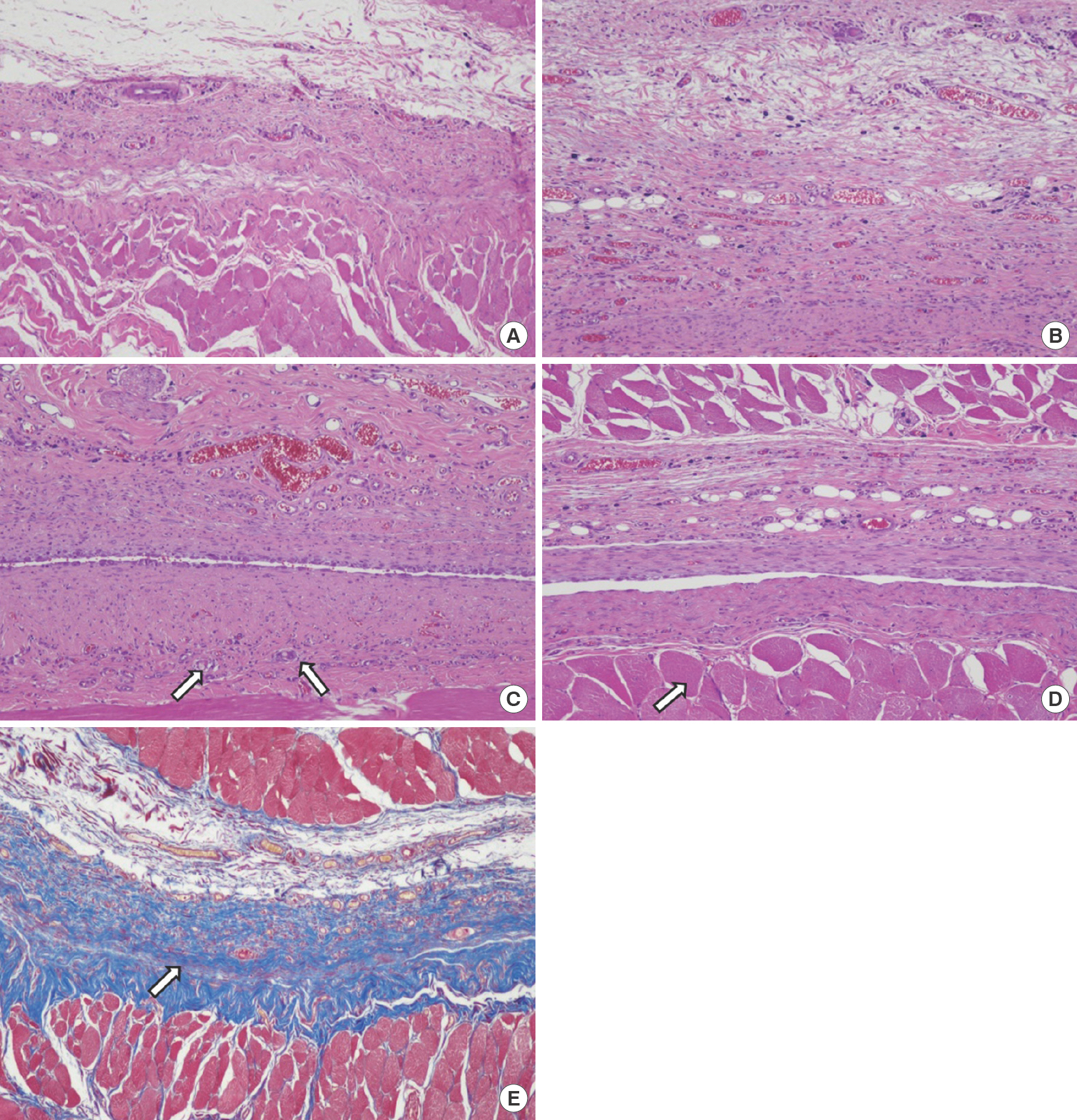
Fig. 9.
Twenty-seven days after wound induction. (A) Control group, complete wound healing was observed with little inflammatory cell infiltration (H&E, ×100). (B) In group A (treated with gentamicin collagen sponge), complete wound healing was observed with little inflammatory cell infiltration (H&E, ×100). (C) Group A, Foreign body giant cell (arrow) increased continuously with decreasing inflammatory cell infiltration (H&E, ×100). (D) Group A, striated muscle repair (arrow) continued until 27 days (H&E, ×100). (E) Extracellular matrix formation (arrow) was similar in both groups, as observed after Masson's trichrome staining (×100).
Study 2: effectiveness of Genta-Coll against nosocomial bacteria
Genta-Coll created a zone of inhibition against all microorganisms except for C. albicans. Although a small number of microorganisms invaded the peripheral regions of the zone of inhibition by 48 hours, compared to the observation made at 24 hours, the diameter of zone of inhibition was similar after 24 and 48 hours. In descending order of zone of inhibition size, the results were as follows: MSSA>MRSA> P. aeruginosa> E. coli> E. faecalis> A. baumannii> C. albicans (Table 5).
Table 5.
Zone of inhibition test of Genta-Coll
Discussion
Wound healing is analyzed based on clinical features as well as biochemical and histological parameters [8]. Wound healing processes can be differentiated into four phases: hemostasis, inflammation, proliferation, and remodeling [9]. Various cells such as keratinocytes, neutrophils, macrophages, lympho-cytes, fibroblasts, and endothelial cells play a significant role in the wound healing process [10]. Therefore, in histological evaluation, the basic components of the wound healing process such as inflammation, fibroblast proliferation, angiogenesis (neovascularization), connective tissue matrix restoration, wound contraction and remodeling, epithelialization, and differentiation must be analyzed [8,11]. The degree of change in wound healing can be observed using a quantitative or a semi-quantitative scoring system. The quantitative measurement helps determine the absolute number of cells and tissue areas, which can be used to derive the quantitative score [12]. Although the quantitative scoring system is highly specific and standardized, it is difficult to score and objectify the exact interval between the two values in most cases [12]. Consequently, semi-quantitative scoring systems are commonly used to measure and express the degree of change between scales in biochemical research [8].
Hemostasis during surgery can be maintained using mechanical, thermal, and chemical methods [1–4,13]. Mechanical methods include direct compression, suturing, stapling, and the use of ligating clips. Thermal methods include electrocautery, use of a hemostatic scalpel, and laser. Chemical methods can be divided into pharmacotherapy and topical hemostatic agents. Topical hemostatic agents can be applied locally on wounds when other methods are not feasible [3]. Studies have tried to identify an ideal hemostatic agent that shows quick and effective hemostatic action and is non-antigenic, absorbable, inexpensive, and easy to use. Unfortunately, since such a perfect agent does not exist yet, surgeons have to select hemostatic agents based on how they affect wound healing.
Topical hemostatic agents can be classified into those that act directly on the clotting cascade, such as thrombin, and those that act passively as a physical structure through contact activation and promotion of platelet aggregation, such as cellulose, collagen, and gelatin [13]. Collagen acts passively as a tissue-derived biomaterial hemostatic agent. As a biomaterial, collagen possesses high tensile strength, high affinity to water, low antigenicity, absorbability in the body, good cell compatibility (adhesion, growth, and migration), and exerts an enhancing effect on tissue regeneration, cell differentiation, and platelet activation. Collagen is available in different shapes for different applications [14]. Collagen-based products facilitate hemostasis through contact activation and the promotion of platelet aggregation through direct contact between blood and collagen [13,15].
Genta-Coll used in this experiment is composed of highly purified equine type 1 collagen sponges that are biologically absorbable and impregnated with gentamicin, enabling its local application. Genta-Coll is also known to promote wound healing and has a hemostatic effect [16]. There are several advantages to applying gentamicin locally. First, when administered locally, even at high concentrations, the serum concentrations remain well below toxic levels, contrary to systemically administered gentamicin. Therefore, renal function is not affected. Second, the risk of developing drug resistance is limited [17,18].
Several studies have examined the benefits of Genta-Coll and its components on wound healing, as noted above. However, the effect of Genta-Coll on wound healing has not been analyzed in terms of histological findings, which is an objective parameter. Therefore, we performed this study to investigate the effect of Genta-Coll on wound healing using biopsy to assess objective histological findings of wound healing in rats. We also performed a second study to investigate the effectiveness of Genta-Coll against nosocomial bacteria to prevent SSIs with the help of a zone of inhibition test. Additionally, we also used a semi-quantitative scoring system to compare each group in this experiment.
In the first study, we analyzed the effect of Genta-Coll on wound healing. We found that foreign body giant cells were abundant in group A (Genta-Coll); however, this parameter was even higher in the control group than in group A on the 7th day of the biopsy. The inflammatory cell infiltration parameter was significantly higher in group A than in the control group; however, there were no significant differences between the two groups. Granulation and neovascularization were continuously high in group A. Fibroblast proliferation and ECM formation was nearly identical in both groups. These findings suggest that the wound healing process was not affected by Genta-Coll in group A, even if foreign body giant cells were slightly abundant compared to those in the control group. In terms of striated muscle repair, the muscle repair rate was high in the control group on the 3rd day of the biopsy. However, a significant increase in muscle regeneration was observed in group A on 7th day's biopsy results. Additionally, muscle regeneration continued until the 27th day of biopsy in group A. The high rate of muscle repair in the control group compared to group A on the 3rd day of biopsy could be attributed either to the high volume of Genta-Coll on the muscle or the high volume of Genta-Coll on the wound itself in group A. Biopsy performed on the 3rd day revealed that Genta-Coll was not absorbed well in the wound and occupied most of the wound. Moreover, the volume of Genta-Coll was nearly the same as that inserted initially. Genta-Coll was not visible to the naked eye during subsequent biopsies, suggesting significant absorption. Although Genta-Coll did not negatively affect wound healing in later stages, it might make healing slightly challenging before absorption, as it fills the wound space. This was probably because the Genta-Coll patch inserted was too large for the wound as the patch was carved using the same punch (8-mm biopsy punch) used to create the muscle defects. We believe that using a Genta-Coll patch smaller than the wound size would have accelerated striated muscle repair in group A compared to the control group by the 3rd day, therby eliminating the volume effect similar to those observed on days 7 and 27.
Our second study assessed the zone of inhibition of Genta-Coll to analyze its effectiveness against nosocomial bacteria. Genta-Coll inhibited all SSI-related pathogens, except for C. albicans. The diameter of the zone of inhibition was similar between 24 and 48 hours, suggesting a continued bactericidal effect of Genta-Coll. These results indicate that Genta-Coll can be an effective prophylactic agent against nosocomial infections in surgical wounds.
This study has several limitations. First, although some data values were not statistically significant in this study, differences between each group were observed on histological evaluation. We believe that statistically significant results can be obtained for all parameters if the study's sample size increases. Second, it is questionable whether the first study results could be generalized to humans, as there are differences between the human and rat physiological structures. Third, the zone of inhibition test by Mueller–Hinton agar plate alone might not be adequate for assessing the antimicrobial activity of Genta-Coll in humans. Two- and three-dimensional in vitro human skin models could be excellent alternatives to verify this issue.
In summary, although group A (Genta-Coll) showed a slightly higher score in foreign body parameters compared to the control group, there was no significant difference between the two groups in terms of inflammatory reactions. However, there was a significant difference between the two groups in terms of striated muscle repair (higher score in group A than in the control group) and continuous muscle repair (higher score in group A over time). This suggests that Genta-Coll may be a good hemostatic agent that can promote faster wound healing and reduced inflammatory reaction as well as cell infiltration irrespective of the mild foreign body reactions. Furthermore, the bactericidal effect may also help prevent SSIs and support wound healing.
Conflict of interest
This work was supported by Wonkwang University in 2021. Young Cheon Na is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.