 |
 |

|
| Aims and Scope |
| About the Journal |
| About the Society (KWMS) |
| Editorial Board |
| Open Access |
| Principles of Transparency and Best Practice |
| Editorial Office |
| Subscriptions |


|
| Aims and Scope |
| About the Journal |
| About the Society (KWMS) |
| Editorial Board |
| Open Access |
| Principles of Transparency and Best Practice |
| Editorial Office |
| Subscriptions |

AbstractBackgroundThe newly-approved Kerecis is a piscine acellular dermal xenograft. This piscine acellular dermal matrix (ADM) has specific bioactive lipid mediators, omega-3 polyunsaturated fatty acids, and has a positive effect on the process of wound healing. This study aimed to explore the utility of this novel material by comparing healing rates, and suggest the proper timing for applying Kerecis.
MethodsPatients who visited the hospital with acute or chronic deep dermal wounds from June 2019 to May 2020 were enrolled in the study. A total of 48 patients were assessed. All wounds in the experimental group (n=16) were treated only once with Kerecis and a non-adherent absorptive foam material (Therasorb) to cover the ADM. In the control group, daily conventional dressings were provided. All wounds sizes were measured with mass-market computer software in a method suggested by the authors for the first time.
ResultsThe mean healing rate proved to be faster in the Kerecis group (P<0.05) versus the control group, and no complications were observed. It was statistically proved that treating burn wounds with the ADM showed better healing rates than the conventional method (P<0.05).
ConclusionThis study establishes that managing wounds with the ADM is likely to heal wounds faster than traditional dressings. In addition, for burn wounds, a prolonged application (10 days vs. 5 days after the onset) showed a better wound healing rate (98.8%±2.5% vs. 67.0%±14.3%, respectively, P=0.029).
IntroductionIn general, wounds are classified into two types depending on the progress of healing: acute and chronic. Improper wound management causes delayed wound healing, which leads to chronicity. A wound is for the most part considered chronic if it has not healed in 4 weeks. Delayed wound healing is also often associated with complications such as infections, scar formation, and incomplete wound healing.
Whether the wound is caused by direct trauma or a burn, proper wound management is one of the essential concerns for early wound healing. In general, wound management contributes to the healing process by protecting the wound fluids, preventing infection, controlling mechanical influences, and influencing the collagen maturation course. Physiologically, growth factors, chemokines, cytokines, and their receptors are critical in normal wound healing [1,2].
Many therapies promoting early wound healing by accentuating spontaneous healing at molecular levels have been recently introduced. Acellular dermal matrix (ADM) is one of such treatments, and it has shown potential for accelerating wound healing. ADM helps wound healing by stimulating angiogenesis, and providing a scaffold for granulation tissue formation [3]. Many kinds of ADM products have been reported to promote tissue regeneration and wound healing, and are in wide clinical use, such as AlloDerm from human skin (LifeCell Crop.), Integra from bovine tendon type I collagen (Integra Lifesciences) and EZ Derm from porcine aldehyde cross-linked dermal collagen (Molnlycke HealthCare).
Most recently, a novel acellular fish-skin graft product, Kerecis Omega3 (Kerecis), has become commercially available. It was already approved from the Food and Drug Administration in the United States in 2016. The material had obtained clearance from the Ministry of Food and Drug Administration in Korea in 2018 as well. We therefore aimed to investigate the utility of this novel material as a dressing material, comparing healing rates between a Kerecis-applied group and a control group. We also attempted to suggest the proper timing to apply Kerecis.
MethodsPatients who visited the emergency department or the outpatient clinic, from June 2019 to May 2020, with acute or chronic wounds of the deep dermal layer were recruited for this study (Fig. 1). We retrospectively surveyed the patient’s age, sex, smoking status, wound location, and categorized the wounds [4]. For acute wounds, we differentiated them by the causes of injury, into either burn or trauma. Patients treated with ADM or conventional dressings were chosen by patient’s preference. The 32 patients in the control group were enrolled in a retrospective cohort during the same period. None of the patients were placed on medical or surgical treatment for faster wound healing. Patients who underwent surgery during the study period or failed to appear for follow-up were excluded. Demographic values in the ADM group were summarized in Tables 1 and 2. There were no significant differences among the patients in other factors such as diabetes, hypertension, smoking, age, sex, and location
Wound dressing using KerecisThe application of Kerecis (Kerecis, Isafjordur, Iceland) was differentiated according to certain criteria. For acute wounds, the ADM was applied around 2 days after the onset of injury when the amount of discharge from each wound sufficiently diminished. For acute burn wounds, it was applied later than acute traumatic wounds, i.e., later than 5 days after the onset, because the discharge from burn wounds is typically more than that of acute wounds. Normally, burn wounds initially have excessive discharge that is likely to impede the effective wound healing of Kerecis.
The Kerecis Omega3 wound matrix was applied on the wound as follows. First, we removed most of the necrotic tissue, measured the size of fresh tissue surface, and cut the material 1 mm to 2 mm larger than the wound to cover the wound margin. The cut material was then bathed in saline solution for 60 seconds at room temperature. The Kerecis sheet was then directly applied on the wound, its rough side face down. Lastly, a non-adherent absorptive foam material (Therasorb 5 mm) was used to cover the ADM (Fig. 2). Wounds were treated only once with Kerecis; those that were managed with other dermal substitutes were excluded. Foam change was performed daily after the ADM was used. In the control group, the wound was covered with the same foam material after applying antibiotic ointment once a day.
Clinical outcomesDigital images, including a ruler (cm scale) within the frame of each image, were photographed with a Canon D750 Camera at a resolution of 6,000×4,000 pixels by a single trained photographer. In order to measure the wound sizes, based on clinical photos, their size and depth were assessed by three different board-certified plastic surgeons (SYW, HGJ, and JHP) (Fig. 3). The three reviewers all evaluated the wound as completely healed when it recovered with full epithelialization, and as healing state when it started to form healthy granulation tissue [5]. To measure the size of the wounds, the digital photos were opened on Adobe Photoshop version 21.2.4 (Adobe, California, LA, USA) and the areas of the initial wounds and epithelialized wounds were calculated by their number of pixels [5-7]. We compared the initial wound size when Kerecis was applied, with the wound size at 7th post-application day. The healing rate was calculated by the change in epithelialization over the course of a week in each group (Fig. 4) [7,8]. We represented and recorded the progress of the wound based on clinical photography. In addition, the number of days until full recovery was recorded. The wounds were also examined for complications such as infection and dermal component loss for further follow-up in 2 months.
This study was approved by the Institutional Review Board of Soonchunhyang University Gumi Hospital (IRB No. 2020-04) and performed in accordance with the principles of the Declaration of Helsinki. Proper informed consent was obtained and each patient was provided with an explanation of the procedure.
AnalysisVariables in such a study are typically presented in values of mean and standard deviation. To compare the mean values, the Mann-Whitney U test was used because the sample size was small. Regression analysis was performed to estimate relationships between a dependent variable and one or more independent variables. Statistical analyses were performed using IMB SPSS Statistic version 27.0 (IBM Corp., Armonk, NY, USA).
ResultsA total of 48 patients with acute and chronic deep dermal-layered wounds were enrolled. Of the 16 patients in the ADM group, 10 were women and six were men with ages ranging from 0 to 89 years (mean, 43.3±25.2 years). There were eight acute burns, and five wounds in the acute stage created by direct trauma such as knife, machine, and glass. Of the other three chronic wounds, one patient had a diabetic foot ulcer on his right fifth toe, one had developed a wound on his right great toe due to venous insufficiency, and one had a pressure sore on her left heel. The average treatment duration in the ADM group was 10.1±5.5 days. The ADM was applied 2 to 3 days (average, 2.40±0.55 days) after the onset of the injury in traumatic acute wounds. For burn wounds, the application was later, from 5 to 12 days (average, 8.00±3.02 days) after the onset.
The Kerecis was not fully absorbed until an average of 5.56±1.60 days after its application. The average healing rate was 77.7%±18.2%, which was measured on when Kerecis was fully absorbed, which was 2 weeks after starting treatment.
The mean healing rate for the traumatic acute wound was 71.4%±18.4% when Kerecis was fully absorbed after its application, and 52.7%±24.1% in the control, which represented no statistical difference (P=0.310). For the burn wounds, the mean healing rate was 86.5%±15.2% in the ADM group and 61.1%±20.7% in the control group (P=0.021). The average healing rate of all wounds with the ADM application in this study was 77.7%±18.2% and 53.3%±22.0% for the control group, demonstrating statistical significance with a P-value of <0.05. The aforementioned information is summarized in Tables 3 and 4, representing the rate of healing for each type of wound.
Meanwhile, for the burn wounds, the mean healing rate for the wounds on which Kerecis was applied over 10 days (n=4) after the onset of injury was 98.8%±1.0%, which is much faster compared to the burn wounds (average, 74.2%±11.7%; n=4) treated with the ADM 5 days after the onset (P<0.05).
Case 1A 43-year-old male patient was injured on the thumb by a machine. He showed an avulsive laceration on his right thumb. The avulsed flap was necrotized and had a deep dermal layer skin defect, 2×1 cm in size. The proximal portion of the defect was primarily closed with nylon number 5-0, and Kerecis was applied on the tip of the defect 3 days after the injury. A week after application, 87.5% epithelialization was observed. The Kerecis remained in place for up to 7 days, promoting wound healing. Additional dressing was performed, and 13 days after the injury, the wound healed completely (Fig. 5).
Case 2A 56-year-old female patient experienced a hot water burn injury. She suffered from a deep second-degree burn on her right lower leg. The wound was nearly 7×2 cm in size and affected the deep dermal layer with a whitish change on the wound bed. The ADM was applied 5 days after the injury, and the wound was epithelialized by 71.4% at 7th day after the first application. The Kerecis was fully absorbed after 5 days. The wound healed completely 14 days after the application of the Kerecis (Fig. 6).
DiscussionADM is widely used in managing various types of wounds. ADMs that are derived from cadaveric or pig skin can trigger a series of autoimmune reactions and transmit viral and bacterial disease, while also being costly [9]. In fact, the main disadvantage of using ADMs among options of wound management is their price (Table 5). However, the cost of Kerecis is relatively low compared to other ADMs.
Kerecis, a newly-approved ADM, is a piscine acellular dermal xenograft, which is derived from the Atlantic cod (Gadus morhua) [10,11]. Fish skin shows architectural similarities to mammalian skin, owing to its molecular structure [9,10,12]. Kerecis maintains its three-dimensional structure composed of glycosaminoglycans, proteoglycans, fibronectin, and growth factors [9]. These lipid mediators are also preserved through the special manufacturing process of Kerecis [10]. Furthermore, the product has specific bioactive lipid mediators called omega-3 polyunsaturated fatty acids, which are a distinct feature of piscine ADM.
It has been reported in previous studies that the omega-3 matrix contributes to the process of wound healing. Baldursson et al. [13] found that treatment with the omega-3 matrix compared to that with porcine matrix (Oasis) caused no seroconversions in autoantibodies and healed much faster. Magnusson et al. [11] concluded that application of the omega-3 matrix in acute complicated wounds shows a 50% mean reduction in wound area in the early stage of healing. Furthermore, studies by Yang et al. [12] proved that the omega-3 matrix was a promising material in managing chronic wounds including diabetic foot ulcers, and a 48% decrease in wound depth was observed after 5 weeks of application of the fish-derived ADM.
Kerecis provides a complex scaffold that gives the optimal environment for a positive host tissue response, characterized by restoration of tissue structure and function, while delivering anti-inflammatory eicosapentanoic acid and docosahexanoic acid type omega-3 fatty acids [12]. Studies have demonstrated that omega-3 fatty acids serve as inflammation regulators [9], promote antibacterial activity against Gram-positive and Gram-negative bacteria, and also antiviral activity against human immunodeficiency virus and herpes simplex viruses [10]. Magnusson et al. [14] reported that fish-skin graft has healthy antibacterial activity compared with human amnion/chorion membrane allograft: the acellular fish-skin grafts can withstand bacterial invasion for up to 48–72 hours. In addition, in vitro studies show that acellular fish-skin grafts furthermore exert an immunomodulatory effect by inhibiting macrophage secretion of the proinflammatory cytokine interleukin 1-beta [13]. Given the literature, we assumed that managing chronic wounds with Kerecis would have a better healing rate than the control group, and proved the efficacy statistically. Further research using Kerecis comprising a large number of chronic wounds is expected to support our findings on its efficacy on chronic wounds.
The microporous structure (i.e., pores of less than 2 nm in diameter) of Kerecis allows autologous cells and capillaries to migrate and promote epithelialization. In addition, it has been described to be useful in covering deep dermal layer wounds, such as wounds sustaining loss of full-thickness skin, for example in injuries where the bone or tendon is exposed [10].
To further verify the efficacy of Kerecis in wound management as was investigated in preceding studies, we found how effectively it acted on a variety of wounds by differentiating the timing of Kerecis application. The right time for application tends to be later in acute burn wounds than in acute traumatic wounds, and is more delayed in chronic wounds compared to acute burn wounds (Table 6). This tendency is attributed to the quantity of discharge from each type of wound. The more discharge, the more meticulous the physicians’ dressings were, and the faster Kerecis was absorbed. Meanwhile, keeping Kerecis longer on the wound surface turned out to not speed up the healing rate (P=0.217). For fast and efficient dressing, it is recommended to start to apply the ADM when discharge is moderate.
In this study, we applied Kerecis in specific wounds of the deep dermal layer and found a decent healing rate of more than 70% when the ADM was fully absorbed.
The application of Kerecis on acute burn wounds showed better progress in terms of wound healing rates compared to acute traumatic wounds, albeit without statistical significance (P=0.435). Overall comparison of healing rate between the two groups demonstrated that using Kerecis (P<0.05) was more efficient. In particular, our stratification analysis revealed that managing burn wounds with the ADM had much better results (P<0.05). In addition, we found that the delayed application of Kerecis on acute burn wounds, later than 10 days, had better outcomes (Table 7). This study controlled confounding variables by randomization during the study period. Independent variables such as personal medical history and the locations and types of wounds were excluded, based on Pearson chi-square test (Tables 1, 2).
Several approaches have been developed and used in clinical and basic research to measure the shape of wounds [5,6,15], from the traditional approach using transparent film to trace the outline of the wound, to use of computer-based software. None has proved to be superior to others in terms of accuracy and efficiency. Although the three-dimensional shape and color of the wound can be obtained and measured using a laser projector and camera [15], it is expensive and not efficient for clinical use [16], also requiring special software and instruments. In this study, we referred to the study by Li et al. [6] for pixel-based wound measuring, and calculated the healing rate on the mass-market Adobe Photoshop program. However, while in Li’s study the authors included much data into a single image and compared them by counting pixels [6,16], we used a reference ruler and the simple program to measure disparate images traced when we first examined the patient and, on the 7th posttreatment day (Fig. 3), introducing a new method of measuring wounds.
The limitations of this study include its small sample size and the attributes of a retrospective study covering a short period of time. Also, the factors influencing healing rate such as nutrition, stress level, alcoholism, and so on were not evaluated because the data in the study was collected from chart reviews [4]. Furthermore, the timing of ADM application differed among the types of injuries, which would have affected the outcomes. In addition, the depth of the wounds was not evaluated by histopathology, and neither were indications for application of the ADM evaluated. A larger number of cases included in a long-term study is required to increase the reliability of the results.
While no previous literature has reported the efficacy of Kerecis for different types of injury, our study provides for the first time different progress rates and durations of healing after the application of Kerecis in acute and chronic wounds. In addition, this article suggests the ideal application timing of Kerecis in acute burn wounds.
ADM is widely used in managing wounds. Its use accelerates wound healing and helps diminish wound complications. A novel acellular fish-skin graft product, Kerecis Omega3, is commercially available. Our study found that managing wounds with the piscine ADM is more effective than with conventional dressings. In addition, we suggest that application of the ADM in managing burn wounds should be delayed until discharges are sufficiently reduced. A 10-day postburn delayed application showed a better wound healing rate than applying the ADM 5 days after the onset.
Conflict of InterestThis work was supported in part by the Soonchunhyang University Research Fund. Otherwise, no potential conflict of interest relevant to this article was reported. Fig. 3.Wound size measurement by three different reviewers. Initial wound size (red line), epithelialized area (blue line), granulation tissue (yellow line). (A) Reviewer 1. (B) Reviewer 2. (C) Reviewer 3. 
Fig. 4.The calculation of the healing rate. (A) The formula of healing rate in this study. (B, C) The size of the wound was assessed by our new method of measuring wound sizes using computer software. SA, initial surface area; SB, day 7 surface area. Raw surface tracing (dotted line), pixel counts (red arrows). 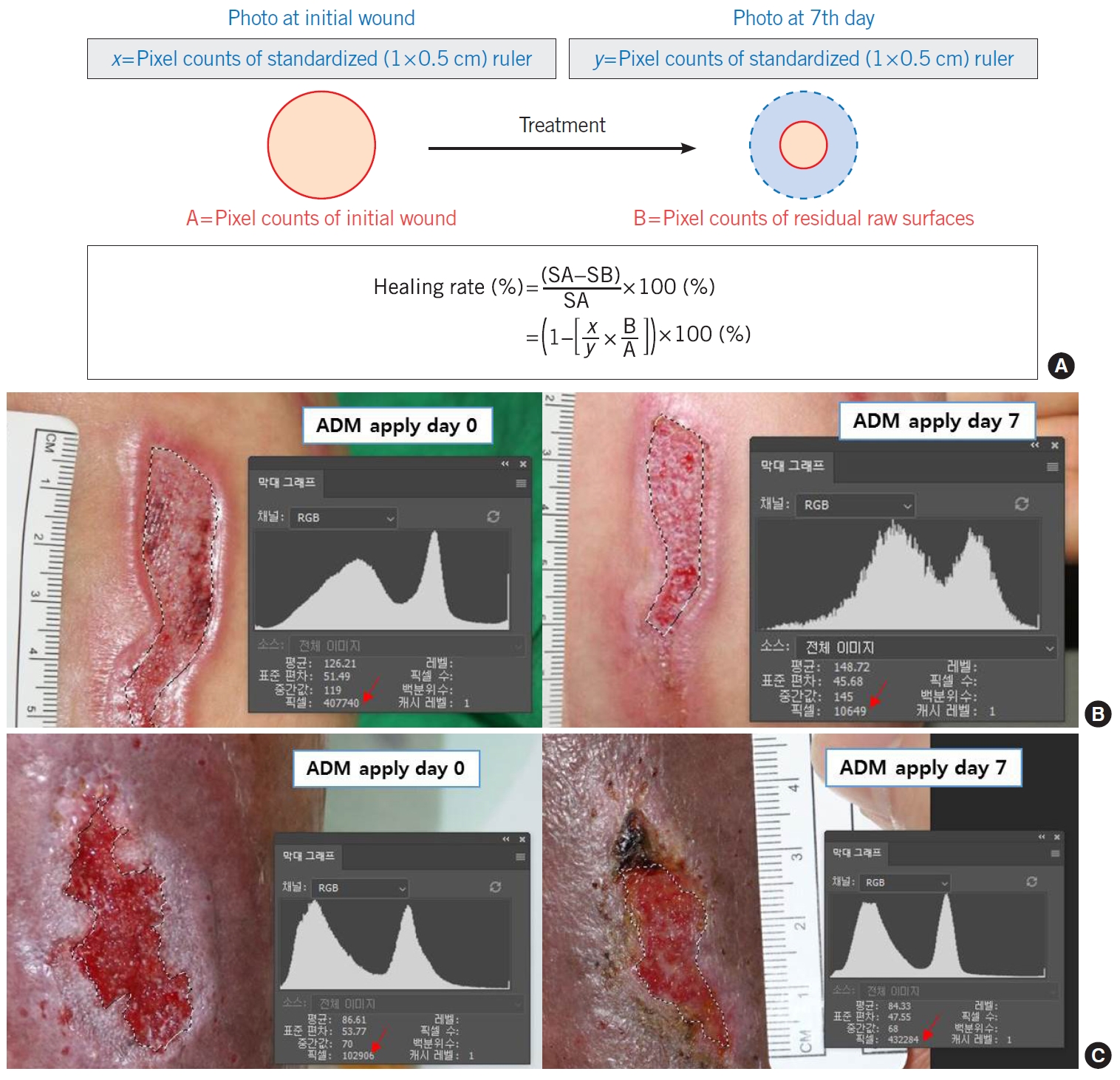
Fig. 5.Acute trauma wound on the thumb. (A) At the time of initial application of the acellular dermal matrix (ADM). (B) Three days after application. (C) Eight days after application, the ADM was fully absorbed. 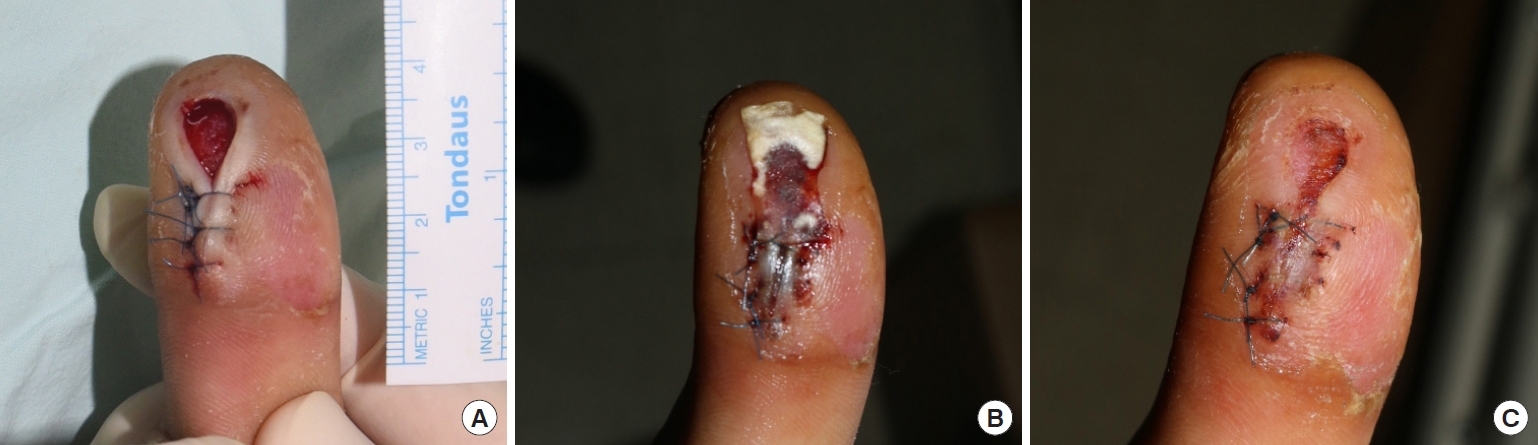
Fig. 6.Acute burn wound on the lower leg. (A) At the time of initial application of the acellular dermal matrix (ADM). (B) Three days after application. (C) Five days after application, the ADM was fully absorbed. 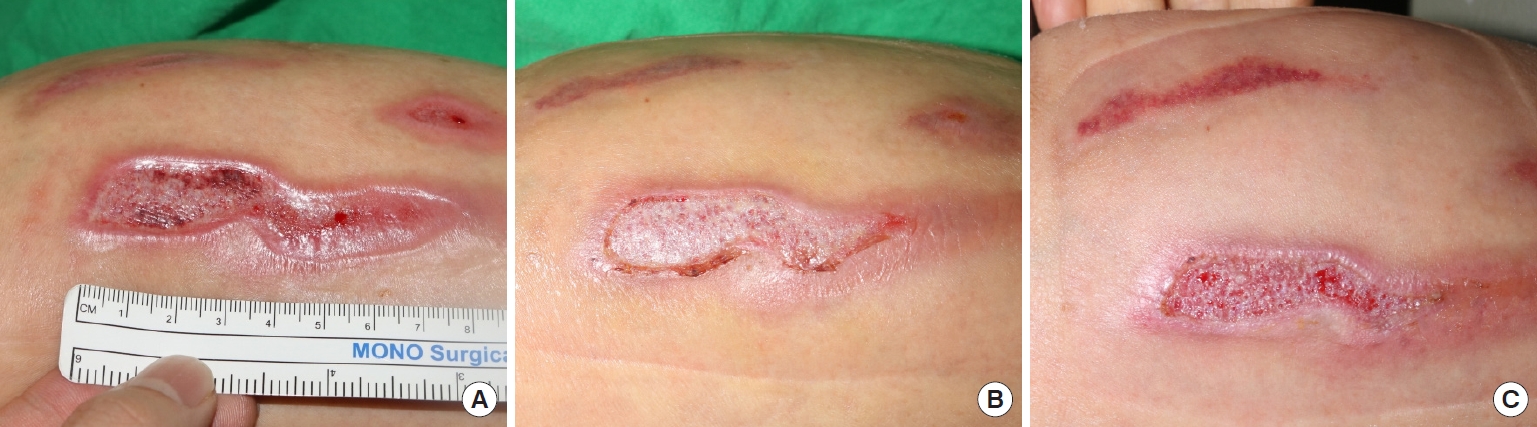
Table 1.Patients with acute or chronic wounds in the Kerecis group
Table 2.Total comparison of each group Table 3.Comparison of healing rate and duration among the causes of injuries
Table 4.Comparison between wound types in the Kerecis group
Table 5.Acellular dermal matrix cost considerations
Table 6.Simple regression analysis References2. Whiddon LL. The treatment of venous ulcers of the lower extremities. Proc (Bayl Univ Med Cent) 2007;20:363-6.
3. Aramwit P. Introduction to biomaterials for wound healing. In: Agren MS, editors. Wound healing biomaterials. Cambridge: Woodhead Publishing; 2016. p. 3-26.
5. Santamaria N, Ogce F, Gorelik A. Healing rate calculation in the diabetic foot ulcer: comparing different methods. Wound Repair Regen 2012;20:786-9.
6. Li PN, Li H, Wu ML, et al. A cost-effective transparency-based digital imaging for efficient and accurate wound area measurement. PLoS One 2012;7:e38069.
7. Lundeberg TC, Eriksson SV, Malm M. Electrical nerve stimulation improves healing of diabetic ulcers. Ann Plast Surg 1992;29:328-31.
8. Cukjati D, Rebersek S, Miklavcic D. A reliable method of determining wound healing rate. Med Biol Eng Comput 2001;39:263-71.
9. Alam K, Jeffery SLA. Acellular fish skin grafts for management of split thickness donor sites and partial thickness burns: a case series. Mil Med 2019;184(Suppl 1):16-20.
10. Dorweiler B, Trinh TT, Dunschede F, et al. The marine Omega3 wound matrix for treatment of complicated wounds: a multicenter experience report. Gefasschirurgie 2018;23(Suppl 2):46-55.
11. Magnusson S, Kjartansson H, Baldursson BT, et al. Acellular fish skin grafts and pig urinary bladder matrix assessed in the collagen-induced arthritis mouse model. Int J Low Extrem Wounds 2018;17:275-81.
12. Yang CK, Polanco TO, Lantis JC 2nd. A prospective, postmarket, compassionate clinical evaluation of a novel acellular fish-skin graft which contains omega-3 fatty acids for the closure of hard-to-heal lower extremity chronic ulcers. Wounds 2016;28:112-8.
13. Baldursson BT, Kjartansson H, Konradsdottir F, et al. Healing rate and autoimmune safety of full-thickness wounds treated with fish skin acellular dermal matrix versus porcine small-intestine submucosa: a noninferiority study. Int J Low Extrem Wounds 2015;14:37-43.
14. Magnusson S, Baldursson BT, Kjartansson H, et al. Regenerative and antibacterial properties of acellular fish skin grafts and human amnion/chorion membrane: implications for tissue preservation in combat casualty care. Mil Med 2017;182:383-8.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||