Introduction
Injury to the hand is one of the difficult problems that often present to plastic surgeons as well as orthopedicians. It is often associated with a soft tissue defect with exposure of muscle, tendon, bone, and neurovascular structure. Covering this defect should be prioritized, as failure to do so may lead to infection and necrosis of the exposed area. The skin coverage methods range from a simple split-thickness skin grafting to more complex flaps. One of the more complicated methods is to use ipsilateral forearm skin to cover the defect. The advantage of using the forearm as a donor area is that because both recipient and donor sites are in a single operative field, the entire procedure can be performed under a single brachial block and postoperative restriction of movement is limited to one limb.
Plastic and reconstructive surgeons would be well advised to develop reliable and versatile skills for covering skin defects under varied circumstances, while also reducing morbidity at the donor site. While distally-based reverse flow flaps such as radial forearm flaps and posterior interosseous artery (PIA) flaps share similar advantages and indications, the radial forearm flap has the distinct drawback that it sacrifices a major artery to the hand. A septocutaneous island flap based on the PIA was originally described by Zancolli and Angrigiani in 1985 [1]. Lu et al. [2] had described it as a reverse flow pedicle flap and Penteado et al. [3] had also previously described the flap in English literature. The reverse PIA flap is based on the anastomosis between the PIA and anterior interosseous artery (AIA) and the reverse flow of blood that runs through them from the volar to dorsal direction. However, it is considered less reliable than the radial forearm flap because of the variation in anatomy of vessels.
The purpose of this study is to evaluate the reverse PIA, or reverse PIA flap, for coverage of soft tissue defect of hand in terms of its safety, reliability, comfort, function and aesthetic appearance of hand.
Methods
Patients with defects presenting over the dorsum or first web space of the hand over a 3-year period from August 2014 to July 2017 were included in this study. A PIA flap was planned, raised and inset over the recipient areas in all such cases.
The study was approved by the Institutional Review Board of Jawaharlal Nehru Medical College, Aligarh Muslim University (IRB No. 2091a/FM/22.01.2016) and performed in accordance with the principles of the Declaration of Helsinki. Proper informed consent was obtained and each patient was provided with an explanation of the procedure and postoperative care.
The results were classified into the categories of good, fair and poor, depending upon the appearance of the flap, coverage of the defect, satisfaction of the patient and postoperative complications. Results were defined as “good” when there was no complication, the flap matched the recipient area and patient was quite satisfied with the outcome. “Fair” was when there was only marginal necrosis, or minimal secondary procedure was required in the postoperative period, or when there was donor site morbidity. Patients in the “poor” category had complete or more than 50% flap loss, requiring a secondary procedure to cover the defect.
A standard PIA flap was raised under loupe magnification after meticulous planning, and brachial or general anesthesia was administered.
Surgical technique
Marking of the flap
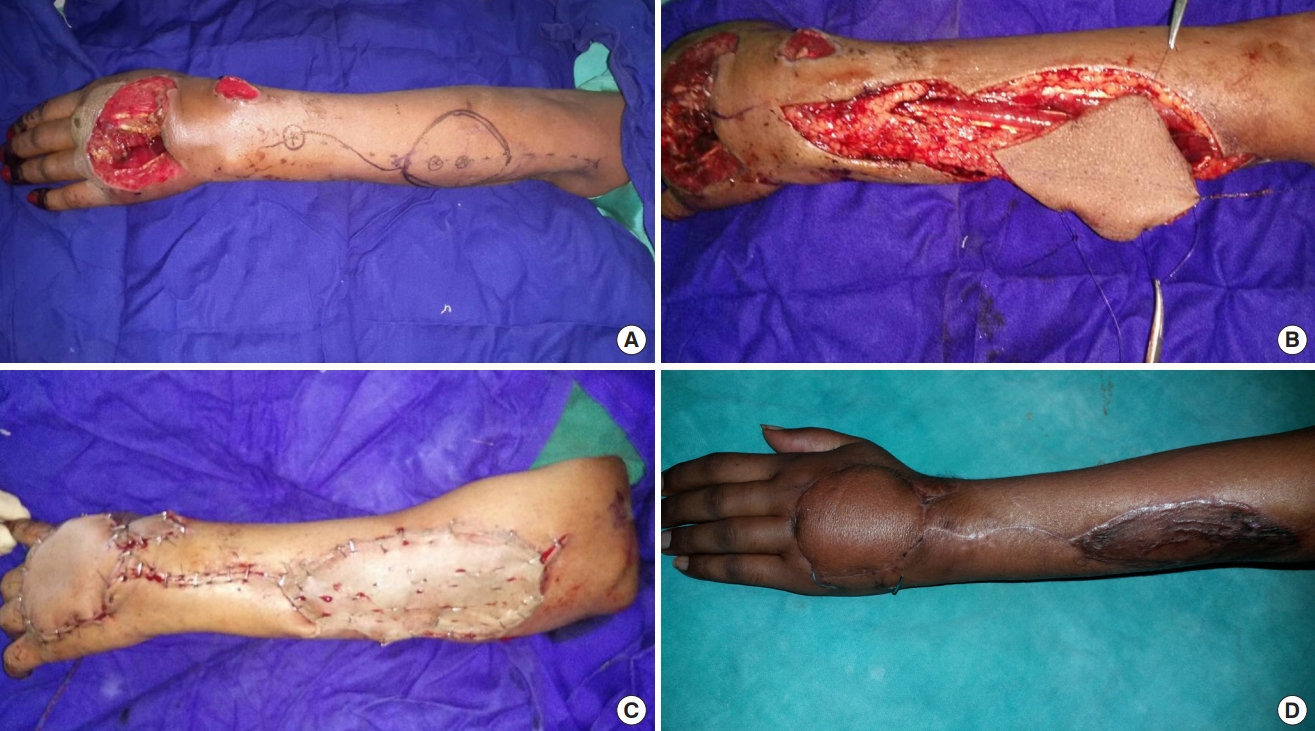
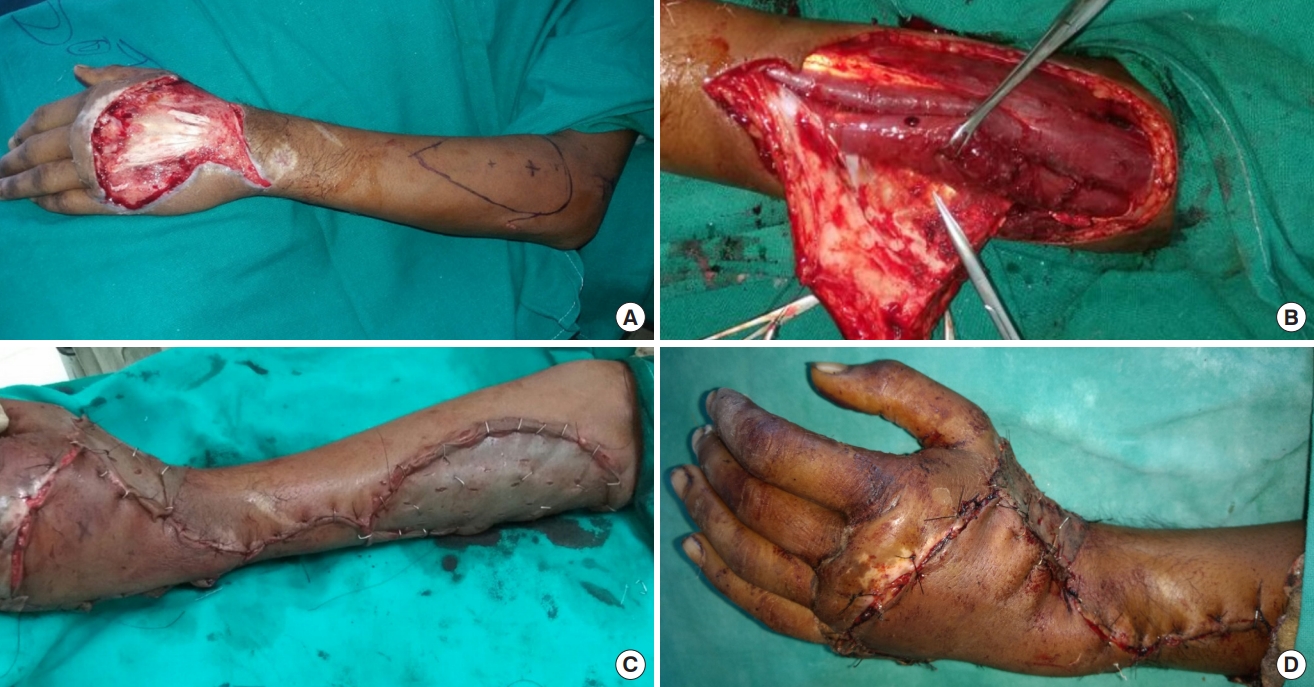
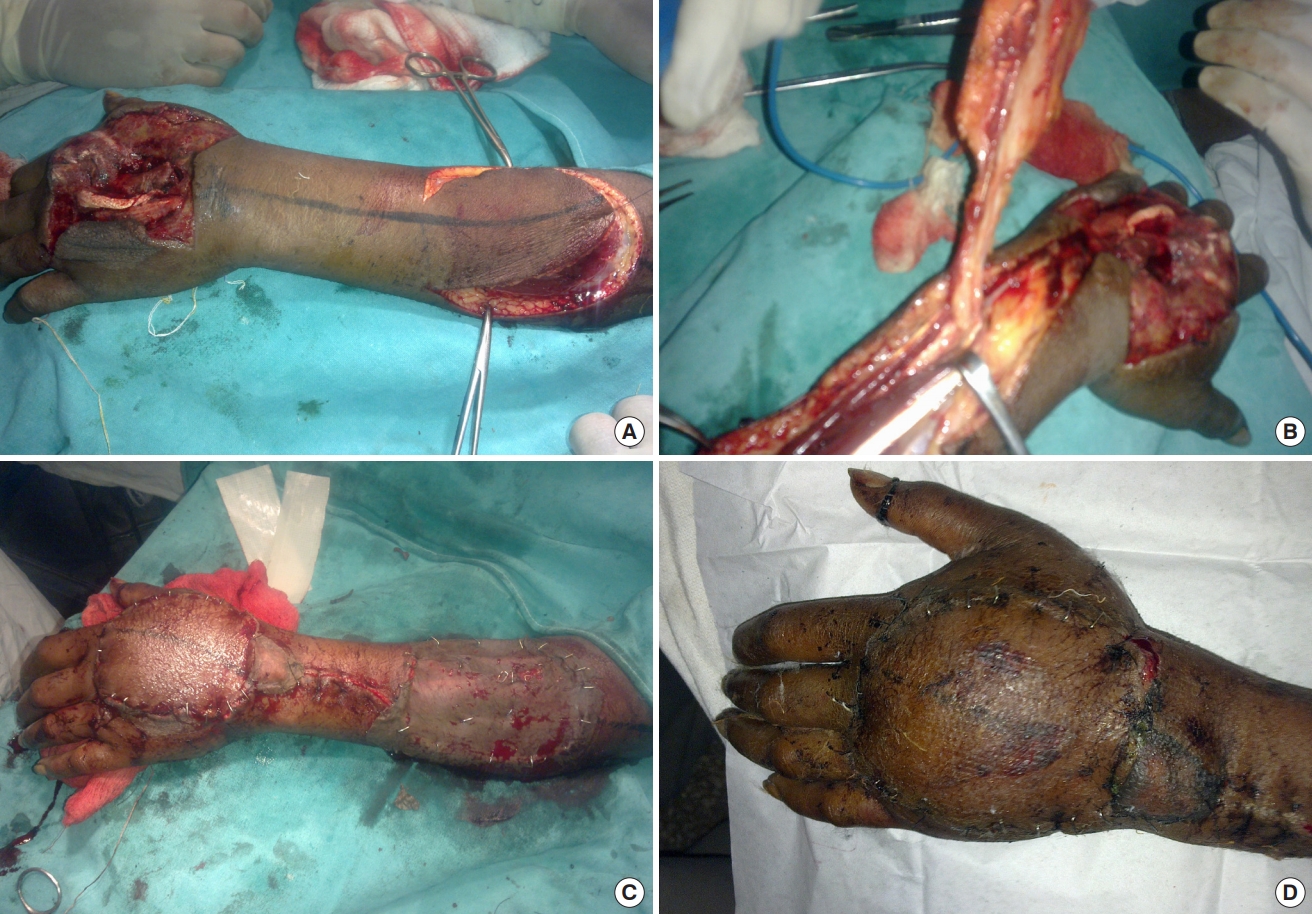
Elbow is flexed at 90° with wrist fully pronated. A straight line is drawn from the lateral epicondyle to distal radioulnar joint. This acts as the vascular axis of the flap. About 2.5 cm proximal to distal radioulnar joint on this axis, a perforator is heard with audio Doppler and marked. This represents the anastomosis between AIA and PIA and is also the pivot point of the flap. The distance from the pivot point to the near edge of the defect is measured; a marking of the same length is made over the proximal vascular axis on the flap. After surgical debridement, the defect is marked, outlined over a sterile linen cloth and extrapolated over the donor area on the proximal forearm.
Tourniquet
The tourniquet is tied over the arm and inflated after keeping the upper limb raised for a few minutes. The limb is not completely exsanguinated to keep the venae comitantes filled with blood for better visibility.
Flap raising
Once the anastomotic perforator between the AIA and PIA is identified, it is dissected before any other measure in the procedure. The pedicle which includes PIA and accompanying venae comitantes is raised with its fascia between extensor carpi ulnaris (ECU) and extensor digiti minimi (EDM). Dissection is proceeded proximally with care taken to avoid injury to the posterior interosseous nerve (PIN) and its branches. The designed flap is then raised first from the ulnar side and cutaneous perforator is visualized in the fascia between EDM and ECU muscles. PIN is identified and preserved beneath these muscles and over the abductor pollicis longus. At least two cutaneous perforators should be identified and included in the flap. The skin flap design may be altered to include more perforators once they are visualized using a loupe in the fascia under the donor skin region. PIA is ligated and cut proximally and the flap is incised on the radial side and raised. The fascia is sutured to the dermis of the flap to avoid shearing of perforators during the raising and manipulating of the flap. After the entire flap is raised, it is inset over the defect and a few drains are inserted beneath the flap. The intervening area is incised to accommodate the pedicle. The donor area is covered with split-thickness skin graft and bolster dressing is tied over it.
Postoperative instructions
The splint is applied with the wrist extended at 20° and the metacarpophalengeal (MCP) joint is flexed 70°. The limb is kept elevated over a coir foam pillow. The flap is monitored regularly to check vascularity by observing color, temperature and using pin prick if required. Occasionally Doppler signals are heard over the pre-marked cutaneous perforators. Intravenous antibiotics are continued for 5 days.
Key points to follow during the procedure
Use Doppler to locate the perforators both at the anastomotic site distally and cutaneous perforators in the designed flap. The surgical procedures should be carried out under loupe magnification. While dissecting around the pedicle, use xylocaine spray to contain the vessels spasming from handling. Always include 2 or more cutaneous perforators. Avoid injury to PIN, particularly during the proximal forearm dissection.
Results
Twelve patients (nine males and three females) were included in this study conducted at our hospital in the department of plastic and reconstructive surgery. Five patients had histories of road traffic accidents, five had injuries from firecrackers and two had suffered injuries from machines. Their mean age was 28.17 years, ranging from 16 to 63 years. Mean width of the defect was 3.38 cm (range, 3–6 cm) and mean length was 9.33 cm (range, 5–12 cm). The mean duration of operation was 150 minutes. In the postoperative period the flaps were assessed clinically by noting temperature, color of the flap, blanching or pricking of the flap. When in doubt, the pre-marked perforator was heard with an audio Doppler using a sterile probe. All flaps survived completely except one, which had marginal necrosis of 1.5 cm, but it healed by secondary intention. In this patient the PIA was located more ulnar beneath the muscle rather than distally between ECU and EDM tendons; therefore, the flap was raised more proximally to increase the reach. The patients’ mean stay in the hospital was 11.5 days, ranging between 9 to 19 days. Eleven out of the 12 cases had good results and only one patient had fair result. All patients were put on early postoperative physiotherapy to prevent joint stiffness. Donor areas in all cases healed well (Tables 1, 2 and Figs. 1-3).
Discussion
The gravity of injuries of the hand, an area with both aesthetic and functional significance, with soft tissue loss has been extensively discussed in literature [4]. As per statistics, the hand is involved in 25% to 48% of reported industrial or agricultural accidents [5]. Soft tissue defects on the hand require good flap cover for proper rehabilitation. There are a few types of flaps from the same forearm which could be used to cover the hand defects with the major advantage of a shorter hospital stay [6]. In patients with injuries to the forearm, abdominal flaps can be used for the coverage [7]. Reverse flow flaps have certain advantages over others, such as the fact that they require only a single-stage procedure, they do not require microvascular anastomosis and also are easy to prepare [8]. They also involve a shorter hospital stay, are well accepted by patients and their caretakers and allow for faster rehabilitation.
Though radial and ulnar artery-based flaps from the forearm have all the advantages described above and require less operating time than the free microvascular flap surgery, they involve sacrifice of a major vessel. To avoid compromising one major vascular axis of the forearm and hand (ulnar or radial artery), a flap based on a PIA is favored [9,10]. It provides a reliable, durable, well-vascularized tissue sufficient to cover the hand defects.
Penteado et al. [3] described the anatomy of this flap and reported 10 cases with retrograde pedicled PIA flaps. In the same paper they advocated that the proximal portion of this flap should not be included in the reverse flow flap because it is supplied by the recurrent interosseous artery.
We have studied this flap in 12 patients in whom reversed PIA flap was used to cover hand defects. Eleven patients had good recovery without any complications, while one had partial necrosis of the flap (up to 30%). In this patient, the cutaneous perforator from PIA at the junction of upper and middle third was found to distally cross the PIN, thereby sacrificing the nerve. Although there were other perforators, the distal portion of the inset flap had decreased vascularity, resulting in necrosis. The area was debrided and skin grafting was done once granulation tissue appeared.
Cheema et al. [11] reported their experience with 64 flaps out of which 88.24% survived completely. The four flaps which failed in their study had skeletonization of the pedicle. We too suggest that the pedicle should have part of fascia around it. Fujiwara et al. [5] also observed the same and suggested inclusion of fascia with the pedicle while raising the flap.
As documented by various authors, the dissection should start distally, and surgeons should look for the anastomotic perforator between the AIA and PIA at least 5 cm proximal to the dorsal wrist crease. We too followed the same principle and found this perforator in all of our cases. The proximal portion of the flap requires meticulous dissection near PIN. Gong and Lu [12] have used PIA flap in severe contractures of the first web space and advised inclusion of proximal perforators and skin paddle to increase the reach of the flap. Rab and Prommersberger [13] used this flap in the first web space reconstruction in two patients, for the wrist in nine patients, for the thumb in two patients and dorsum of hand in eight patients with excellent results.
While surgeons should be aware of anatomical variations in the vessels, few authors have reported their experiences regarding the variations. During their dissection, Penteado et al. [3] did not find PIA beyond the middle third of the forearm in four cases and no anastomotic perforator was found in one case. Costa et al. reported that the PIA was always present in the septum in 25 cases [14,15]. Angrigiani et al. [1] noted the narrowing of PIA beyond the middle third of the forearm in 74 out of 80 cases. In two cases out of 36, Buchler and Frey [16] did not find PIA beyond the middle third of the forearm. We also found one anomaly in a patient who had partial loss of the flap. In this patient, the nerve had crossed the dominant perforator distally. Aesthetically this flap is well accepted because the skin of the forearm closely matches the skin of the dorsal aspect of hand. Eleven out of 12 patients in our study were quite satisfied with the cosmetic outcome.
In order to obtain optimum results, we suggest that surgeons always look for perforators at both beneath the skin paddle and at the anastomosing site with an audio Doppler. If good signals are heard then the dissection may be carried out as planned. More fascia should always be included near the pivot point and if the flap is to be of a significant size, at least two perforators should be included. If PIN is located distal to the proximal skin paddle perforator then another perforator should be looked for and included in the flap design, because in this scenario the proximal perforator is sacrificed to avoid injuring the PIN. The vessels tend to go in spasm during handling and this can be reversed with the help of 15% xylocaine spray [17]. The limb should be kept elevated and splinted in the postoperative period.
PIA flap is a safe, reliable and versatile option for coverage of hand defects, especially over the dorsum and first web space, provided it is well planned with an anastomotic perforator marked with Doppler (both pre- and intraoperatively) and the surgery is done under loupe magnification. The vasospasm of the vessels should be contained with occasional spraying of xylocaine.
The entire procedure requires a shorter stay in the hospital and allows for an earlier recovery of normal functions, as well as more comfort for patients during the postoperative period relative to a groin or thoraco-umbilical flap. It also prevents development of contractures observed in simple split-thickness skin grafting.